 | Nuclear Chemistry
Examples of
Multiple Choice Questions
|
- 1.
- The "magic numbers" for atoms are
- (a) numbers of electrons that confer atomic stability.
- (b) numbers of protons and/or neutrons that confer nuclear stability.
- (c) n/p ratios that confer nuclear stability.
- (d) atomic masses that confer nuclear stability.
- (e) atomic masses that indicate fissile isotopes.
- 2.
- The actual mass of a 37Cl atom is 36.966 amu. Calculate the mass defect (amu/atom) for a 37Cl atom.
- (a) 0.623 amu
- (b) 0.388 amu
- (c) 0.263 amu
- (d) 0.341 amu
- (e) none of these
- 3.
- The mass defect for an isotope was found to be 0.410 amu/atom. Calculate the binding energy in kJ/mol of atoms. (1 J = 1 kg
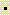 m2/s2)
m2/s2)
- (a) 3.69 x 1010 kJ/mol
- (b) 1.23 x 1020 kJ/mol
- (c) 3.69 x 1013 kJ/mol
- (d) 1.23 x 103 kJ/mol
- (e) 1.23 x 1023 kJ/mol
- 4.
- Calculate the binding energy per nucleon (in units of MeV) for 9Be,
for which the atomic mass is 9.01219 amu. Particle masses in amu are:
proton = 1.007277; neutron = 1.008665; electron = 0.0005486. Conversion
factor for E = mc2 is 931 MeV/amu.
- (a) 6.46 MeV
- (b) 6.33 MeV
- (c) 6.23 MeV
- (d) 11.39 MeV
- (e) 56.93 MeV
- 5.
- Which isotope below has the highest nuclear binding energy per gram? No calculation is necessary.
- (a) 4He
- (b) 16O
- (c) 32S
- (d) 55Mn
- (e) 238U
- 6.
- Which of the following statements is incorrect?
- (a) Mass defect is the amount of matter that would be
converted into energy if a nucleus were formed from initially separated
protons and neutrons.
- (b) Nuclear binding energy is the energy released in the formation of an atom from subatomic particles.
- (c) Nuclei with highest binding energies are the most stable nuclei.
- (d) Einstein postulated the Theory of Relativity in which he stated that matter and energy are equivalent.
- (e) Mass number is the sum of all protons and electrons in an atom.
- 7.
- A positron has a mass number of _____, a charge of _____, and a mass equal to that of a(an) _____.
- (a) 0, 1+, proton
- (b) 1, 2+, proton
- (c) 0, 1+, electron
- (d) 1, 2+, electron
- (e) 0, 0, proton
- 8.
- Emission of which one of the following leaves both atomic number and mass number unchanged?
- (a) positron
- (b) neutron
- (c) alpha particle
- (d) gamma radiation
- (e) beta particle
- 9.
- Which type of radiation is the least penetrating?
- (a) alpha
- (b) beta
- (c) gamma
- (d) x-ray
- (e) neutron
- 10.
- A radioisotope of argon, 35Ar, lies below the "band of stability: (n/p ratio too low). One would predict that it decays via _____.
- (a) neutron emission
- (b) beta emission
- (c) positron emission
- (d) alpha emission
- (e) fission
- 11.
- A Geiger-Muller tube is a _____ .
- (a) gas ionization detector
- (b) cloud chamber
- (c) fluorescence detector
- (d) spectrophotometer
- (e) photographic detector
- 12.
- The half life of 231Pa is 3.25 x 104 years. How much of an initial 10.40 microgram sample remains after 3.25 x 105 years?
- (a) 0.0102 micrograms
- (b) 0.240 micrograms
- (c) 2.18 micrograms
- (d) 0.0240 micrograms
- (e) 1.04 micrograms
- 13.
- Consider
the case of a radioactive element X which decays by electron (beta)
emission with a half-life of 4 days to a stable nuclide of element Z.
Which of the following statements is CORRECT?
- (a) After 8 days the sample will consist of one-fourth element Z and three-fourths element X.
- (b) Element Z will weigh exactly the same as element X when decay is complete (weighed to an infinite number of significant figures).
- (c) 2.0 g of element X is required to produce 1.5 g of element Z after 8 days (to 2 significant figures).
- (d) If element X as an atomic number equal to n, then element X has an atomic number equal to n-1.
- (e) None of the above.
- 14.
- Carbon-11
is a radioactive isotope of carbon. Its half-life is 20 minutes. What
fraction of the initial number of C-11 atoms in a sample will have
decayed away after 80 minutes?
- (a) 1/16
- (b) 1/8
- (c) 1/4
- (d) 7/8
- (e) 15/16
- 15.
- How old is a bottle of wine if the tritium (3H) content (called activity) is 25% that of a new wine? The half-life of tritium is 12.5 years.
- (a) 1/4 yr
- (b) 3.1 yr
- (c) 25 yr
- (d) 37.5 yr
- (e) 50 yr
- 16.
- A
Geiger counter registered 1000 counts/second from a sample that
contained a radioactive isotope of polonium. After 5.0 minutes, the
counter registered 281 counts/second. What is the half-life of this
isotope in seconds?
- (a) 87
- (b) 110
- (c) 164
- (d) 264
- (e) 2.18
- 17.
- The 14C
activity of some ancient Peruvian corn was found to be 10
disintegrations per minute per gram of C. If present-day plant life
shows 15 dpm/g, how old is the Peruvian corn? The half-life of 14C is 5730 years.
- (a) 1455 years
- (b) 1910 years
- (c) 3350 years
- (d) 3820 years
- (e) 9080 years
- 18.
- Which of the following describes what occurs in the fission process?
- (a) A heavy nucleus is fragmented into lighter ones.
- (b) A neutron is split into a neutron and proton.
- (c) Two light nuclei are combined into a heavier one.
- (d) A proton is split into three quarks.
- (e) A particle and anti-particle appear in an area of high energy density.
- 19.
- Which of the following statements about nuclear fission is always correct?
- (a) Very little energy is released in fission processes.
- (b) Nuclear fission is an energetically favorable process for heavy atoms.
- (c) Due to its instability, 56Fe readily undergoes fission.
- (d) In fission reactions, a neutron is split into a proton and an electron.
- (e) All nuclear fission reactions are spontaneous.
- 20.
- Which one of the following would be most likely to undergo thermonuclear fusion?
- (a) 2H
- (b) 4He
- (c) 56Fe
- (d) 141Ba
- (e) 235U
- 21.
- Which one of the following statements about nuclear reactions is false?
- (a) Particles within the nucleus are involved.
- (b) No new elements can be produced.
- (c) Rate of reaction is independent of the presence of a catalyst.
- (d) Rate of reaction is independent of temperature.
- (e) They are often accompanied by the release of enormous amounts of energy.
- 22.
- Complete and balance the following equation. The missing term is _____ .
239Pu + alpha particle
 _____ + neutron
_____ + neutron
- (a) 2 115Ag
- (b) 2 106Rh
- (c) 235U
- (d) 233Pa
- (e) 242Cm
- 23.
- When 59Cu undergoes positron emission, what is the immediate nuclear product?
- (a) 59Ni
- (b) 58Ni
- (c) 58Cu
- (d) 59Zn
- (e) 58Zn
- 24.
- As a result of the process of electron capture ("K-capture") by 211At, the new isotope formed is:
- (a) 210At
- (b) 212At
- (c) 211Po
- (d) 211Rn
- (e) 207Bi
- 25.
- When 235U is bombarded with one neutron, fission occurs and the products are three neutrons, 94Kr, and _____ .
- (a) 139Ba
- (b) 141Ba
- (c) 139Ce
- (d) 139Xe
- (e) 142I
Answers:
1. (b) 2. (d) 3. (a) 4. (a) 5. (d) 6. (e) 7. (c) 8. (d) 9. (a) 10. (c) 11. (a) 12. (a) 13. (c) 14. (e) 15. (c) 16. (c) 17. (c) 18. (a) 19. (b) 20. (a) 21. (b) 22. (e) 23. (a) 24. (c) 25. (a)

 Click here to return to the top.
Click here to return to the top.

 Choose your next chapter:
Choose your next chapter:
| Fundamentals of Chemistry
| Chemical Formulas & Composition Stoichiometry
| Chemical Equations & Rxn Stoichiometry
| Types of Chemical Reactions
|
| Atomic Structure
| Chemical Periodicity
| Chemical Bonding
| Molecular Structure/Covalent Bonding Theories| Molecular Orbital Theory |
| Acids/Bases/Salts - Theory & Rxns
| Acids/Bases/Salts - Calculations (including balancing redox rxns)
| Gases
| Solids & Liquids
| Solutions |
| Thermodynamics
| Kinetics
| Equilibrium
| Aqueous Equilibrium - Acids/Bases/Salts
| Aqueous Equilibrium - Buffers & Titrations
|
| Aqueous Equilibrium - Slightly Soluble Salts
| Electrochemistry
| Metallurgy
| Metal Properties & Rxns
| Nonmetals & Metalloids
|
| Coordination Compounds
| Nuclear Chemistry
| Organic Chem - Formulas/Names/Properties
| Organic Chem - Shapes/Rxns/Biopolymers |

 To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.
To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.


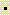 m2/s2)
m2/s2)
 _____ + neutron
_____ + neutron
 Click here to return to the top.
Click here to return to the top.
 Choose your next chapter:
Choose your next chapter: 
 To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.
To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.