 | Thermodynamics
Examples of
Multiple Choice Questions
|
- 1.
- Which one of the following thermodynamic quantities is not a state function?
- (a) Gibbs free energy
- (b) enthalpy
- (c) entropy
- (d) internal energy
- (e) work
- 2.
- At a
constant temperature, an ideal gas is compressed from 6.0 liters to 4.0
liters by a constant external pressure of 5.0 atm. How much work is
done on the gas?
- (a) w = +10 liter atm
- (b) w = -10 liter atm
- (c) w = +30 liter atm
- (d) w = -30 liter atm
- (e) The answer cannot be calculated.
- 3.
- A
system suffers an increase in internal energy of 80 J and at the same
time has 50 J of work done on it. What is the heat change of the
system?
- (a) +130 J
- (b) +30 J
- (c) -130 J
- (d) -30 J
- (e) 0 J
- 4.
- A 5.000 g sample of methanol, CH3OH,
was combusted in the presence of excess oxygen in a bomb calorimeter
conaining 4000 g of water. The temperature of the water increased from
24.000 oC to 29.765 oC. The heat capacity of the calorimeter was 2657 J/oC. The specific heat of water is 4.184 J/goC. Calculate
 E for the reaction in kJ/mol.
E for the reaction in kJ/mol.
- (a) -314 kJ/mol
- (b) -789 kJ/mol
- (c) -716 kJ/mol
- (d) -121 kJ/mol
- (e) -69.5 kJ/mol
- 5.
- A coffee cup calorimeter having a heat capacity of 451 J/oC was used to measure the heat evolved when 0.0300 mol of NaOH(s) was added to 1000 mL of 0.0300 M HNO3 initially at 23.000 oC. The temperature of the water rose to 23.639 oC. Calculate
 H (in kJ/mol NaNO3) for this reaction. Assume the specific heat of the final solution is 4.18 J/goC; the density of each solution is 1.00 g/mL; and the addition of solid does not appreciably affect the volume of the solution.
HNO3(aq) + NaOH(s)
H (in kJ/mol NaNO3) for this reaction. Assume the specific heat of the final solution is 4.18 J/goC; the density of each solution is 1.00 g/mL; and the addition of solid does not appreciably affect the volume of the solution.
HNO3(aq) + NaOH(s)  NaNO3(aq) + H2O(l)
NaNO3(aq) + H2O(l)
- (a) -63.7 kJ/mol
- (b) -151 kJ/mol
- (c) -2.55 kJ/mol
- (d) -81.4 kJ/mol
- (e) -98.6 kJ/mol
- 6.
- The
 Ho for the following reaction at 298 K is -36.4 kJ.
1/2 H2(g) + 1/2 Br2(l)
Ho for the following reaction at 298 K is -36.4 kJ.
1/2 H2(g) + 1/2 Br2(l)  HBr(g)
Calculate
HBr(g)
Calculate  Eo at 298 K. The universal gas constant, R, is 8.314 J/mol K.
Eo at 298 K. The universal gas constant, R, is 8.314 J/mol K.
- (a) -35.2 kJ
- (b) +35.2 kJ
- (c) -36.4 kJ
- (d) -37.6 kJ
- (e) +37.6 kJ
- 7.
- Calculate the amount of work done for the conversion of 1.00 mole of Ni to Ni(CO)4 in the reaction below, at 75oC. Assume that the gases are ideal. The value of R is 8.31 J/mol
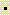 K.
Ni(s) + 4 CO (g)
K.
Ni(s) + 4 CO (g)  Ni(CO)4(g)
Ni(CO)4(g)
- (a) 1.80 x 103 J
- (b) 8.68 x 103 J
- (c) -1.80 x 103 J
- (d) -8.68 x 103 J
- (e) -494 J
- 8.
- All of the following have a standard heat of formation value of zero at 25oC and 1.0 atm except:
- (a) N2(g)
- (b) Fe(s)
- (c) Ne(g)
- (d) H(g)
- (e) Hg(l)
- 9.
- For which of the following reactions would the
 Ho for the reaction be labeled
Ho for the reaction be labeled  Hfo?
Hfo?
- (a) Al(s) + 3/2 H2(g) + 3/2 O2(g)
 Al(OH)3(s)
Al(OH)3(s)
- (b) PCl3(g) + 1/2 O2(g)
 POCl3(g)
POCl3(g)
- (c) 1/2 N2O(g) + 1/4 O2(g)
 NO(g)
NO(g)
- (d) CaO(s) + SO2(g)
 CaSO3(s)
CaSO3(s)
- (e) The
 Ho for all these reactions would be labeled
Ho for all these reactions would be labeled  Hfo.
Hfo.
- 10.
- Calculate
 Ho for the reaction:
Na2O(s) + SO3(g)
Ho for the reaction:
Na2O(s) + SO3(g)  Na2SO4(g)
given the following information:
Na2SO4(g)
given the following information:
|  Ho Ho |
(1) Na(s) + H2O(l)  NaOH(s) + 1/2 H2(g) NaOH(s) + 1/2 H2(g) | -146 kJ |
(2) Na2SO4(s) + H2O(l)  2NaOH(s) + SO3(g) 2NaOH(s) + SO3(g) | +418 kJ |
(3) 2Na2O(s) + 2H2(g)  4Na(s) + 2H2O(l) 4Na(s) + 2H2O(l) | +259 kJ |
- (a) +255 kJ
- (b) -435 kJ
- (c) -581 kJ
- (d) +531 kJ
- (e) -452 kJ
- 11.
- Calculate
 Horxn for the following reaction at 25.0 oC:
Horxn for the following reaction at 25.0 oC:
| Fe3O4(s) | + | CO(g) |  | 3FeO(s) | + | CO2(g) |
 Hfo (kJ/mol) Hfo (kJ/mol) | -1118 | | -110.5 | | -272 | | -393.5 |
- (a) -263 kJ
- (b) 54 kJ
- (c) 19 kJ
- (d) -50 kJ
- (e) 109 kJ
- 12.
- Calculate the standard heat of formation,
 Hfo, for FeS2(s), given the following information:
2FeS2(s) + 5O2(g)
Hfo, for FeS2(s), given the following information:
2FeS2(s) + 5O2(g)  2FeO(s) + 4SO2(g)
2FeO(s) + 4SO2(g)
 Horxn = -1370 kJ
Horxn = -1370 kJ
 Hfo for SO2(g) = -297 kJ/mol
Hfo for SO2(g) = -297 kJ/mol
 Hfo for FeO(s) = -268 kJ/mol
Hfo for FeO(s) = -268 kJ/mol
- (a) -177 kJ
- (b) -1550 kJ
- (c) -774 kJ
- (d) -686 kJ
- (e) +808 kJ
- 13.
- Estimate the heat of reaction at 298 K for the reaction shown, given the average bond energies below.
Br2(g) + 3F2(g)
 2BrF3(g)
2BrF3(g)
| Bond | | Bond Energy |
| Br-Br | | 192 kJ |
| F-F | | 158 kJ |
| Br-F | | 197 kJ |
- (a) -516 kJ
- (b) -410 kJ
- (c) -611 kJ
- (d) -665 kJ
- (e) -720 kJ
- 14.
- What is the standard entropy change of the reaction below at 298 K with each compound at the standard pressure?
| N2(g) | + | 3H2(g) |  | 2NH3(g) |
| So298 (J/mol K) | 191.5 | | 130.6 | | 192.3 | | |
- (a) -198.7 J/K
- (b) 76.32 J/K
- (c) 303.2 J/K
- (d) -129.7 J/K
- (e) 384.7 J/K
- 15.
- The entropy will usually increase when
- I. a molecule is broken into two or more smaller molecules.
- II. a reaction occurs that results in an increase in the number of moles of gas.
- III. a solid changes to a liquid.
- IV. a liquid changes to a gas.
- (a) I only
- (b) II only
- (c) III only
- (d) IV only
- (e) I, II, III, and IV
- 16.
- Calculate
 Go for the reaction given the following information:
2SO2(g) + O2(g)
Go for the reaction given the following information:
2SO2(g) + O2(g)  2SO3(g)
2SO3(g)
 Gfo for SO2(g) = -300.4 kJ/mol
Gfo for SO2(g) = -300.4 kJ/mol
 Gfo for SO3(g) = -370.4 kJ/mol
Gfo for SO3(g) = -370.4 kJ/mol
- (a) -70.0 kJ
- (b) +70.0 kJ
- (c) -670.8 kJ
- (d) -140.0 kJ
- (e) +140.0 kJ
- 17.
- For the following reaction at 25oC,
 Ho = +115 kJ and
Ho = +115 kJ and  So = +125 J/K. Calculate
So = +125 J/K. Calculate  Go for the reaction at 25o.
SBr4(g)
Go for the reaction at 25o.
SBr4(g)  S(g) + 2Br2(l)
S(g) + 2Br2(l)
- (a) +152 kJ
- (b) -56.7 kJ
- (c) +77.8 kJ
- (d) +37.1 kJ
- (e) -86.2 kJ
- 18.
- The heat of vaporization of freon, CCl2F2, is 17.2 kJ/mol at 25oC. What is the change of entropy for one mole of liquid freon when it vaporizes at 25oC? (Hint: The vaporization process is at equilibrium and what is true for
 G at equilibrium?)
G at equilibrium?)
- (a) 57.7 J/K
- (b) 0.688 J/K
- (c) 5.13 x 103 kJ/K
- (d) 3.16 J/K
- (e) 239 J/K
- 19.
- Estimate the boiling point of Br2(l) (
 H = 30.9 kJ;
H = 30.9 kJ;  S = 93.0 J/K).
Br2(l)
S = 93.0 J/K).
Br2(l)  Br2(g)
Br2(g)
- (a) 85oC
- (b) 373oC
- (c) 177oC
- (d) 59oC
- (e) 44oC
- 20.
- For the reaction, A + B
 C,
C,  Ho = +30 kJ;
Ho = +30 kJ;  So = +50 J/K.
So = +50 J/K.
Therefore the reaction is:
- (a) spontaneous at all temperatures.
- (b) nonspontaneous at all temperatures.
- (c) spontaneous at temperatures less than 600 K.
- (d) spontaneous at temperatures greater than 600 K.
- (e) spontaneous only at 25oC.
- 21.
- How much heat is absorbed in the complete reaction of 3.00 grams of SiO2 with excess carbon in the reaction below?
 Ho for the reaction is +624.7 kJ.
SiO2(s) + 3C(s)
Ho for the reaction is +624.7 kJ.
SiO2(s) + 3C(s)  SiC(s) + 2CO(g)
SiC(s) + 2CO(g)
- (a) 366 kJ
- (b) 1.13 x 105 kJ
- (c) 5.06 kJ
- (d) 1.33 x 104 kJ
- (e) 31.2 kJ
- 22.
- The standard heat of combustion of ethanol, C2H5OH, is 1372 kJ/mol ethanol. How much heat (in kJ) would be liberated by completely burning a 20.0 g sample?
- (a) 686 kJ
- (b) 519 kJ
- (c) 715 kJ
- (d) 597 kJ
- (e) 469 kJ
- 23.
- Which statement is incorrect?
- (a) At constant pressure,
 H =
H =  E + P
E + P V
V
- (b) The thermodynamic symbol for entropy is S.
- (c) Gibbs free energy is a state function.
- (d) For an endothermic process,
 H is negative.
H is negative.
- (e) If the work done by the system is greater than the heat absorbed by the system,
 E is negative.
E is negative.
- 24.
- Which statement is false?
- (a) The thermodynamic quantity most easily measured in a "coffee cup" calorimeter is
 H.
H.
- (b) No work is done in a reaction occurring in a bomb calorimeter.
- (c)
 H is sometimes exactly equal to
H is sometimes exactly equal to  E.
E.
- (d)
 H is often nearly equal to
H is often nearly equal to  E.
E.
- (e)
 H is equal to
H is equal to  E for the reaction:
2H2(g) + O2(g)
E for the reaction:
2H2(g) + O2(g)  2H2O(g)
2H2O(g)
Answers:
1. (e) 2. (a) 3. (b) 4. (c) 5. (e) 6. (d) 7. (b) 8. (d) 9. (a) 10. (c) 11. (c) 12. (a) 13. (a) 14. (a) 15. (e) 16. (d) 17. (c) 18. (a) 19. (d) 20. (d) 21. (e) 22. (d) 23. (d) 24. (e)

 Click here to return to the top.
Click here to return to the top.

 Choose your next chapter:
Choose your next chapter:
Fundamentals of Chemistry
| Chemical Formulas & Composition Stoichiometry
| Chemical Equations & Rxn Stoichiometry
| Types of Chemical Reactions
|
| Atomic Structure
| Chemical Periodicity
| Chemical Bonding
| Molecular Structure/Covalent Bonding Theories| Molecular Orbital Theory |
| Acids/Bases/Salts - Theory & Rxns
| Acids/Bases/Salts - Calculations (including balancing redox rxns)
| Gases
| Solids & Liquids
| Solutions |
| Thermodynamics
| Kinetics
| Equilibrium
| Aqueous Equilibrium - Acids/Bases/Salts
| Aqueous Equilibrium - Buffers & Titrations
|
| Aqueous Equilibrium - Slightly Soluble Salts
| Electrochemistry
| Metallurgy
| Metal Properties & Rxns
| Nonmetals & Metalloids
|
| Coordination Compounds
| Nuclear Chemistry
| Organic Chem - Formulas/Names/Properties
| Organic Chem - Shapes/Rxns/Biopolymers |

 To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.
To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.


 E for the reaction in kJ/mol.
E for the reaction in kJ/mol.
 H (in kJ/mol NaNO3) for this reaction. Assume the specific heat of the final solution is 4.18 J/goC; the density of each solution is 1.00 g/mL; and the addition of solid does not appreciably affect the volume of the solution.
H (in kJ/mol NaNO3) for this reaction. Assume the specific heat of the final solution is 4.18 J/goC; the density of each solution is 1.00 g/mL; and the addition of solid does not appreciably affect the volume of the solution.
 NaNO3(aq) + H2O(l)
NaNO3(aq) + H2O(l) Ho for the following reaction at 298 K is -36.4 kJ.
Ho for the following reaction at 298 K is -36.4 kJ.
 HBr(g)
HBr(g) Eo at 298 K. The universal gas constant, R, is 8.314 J/mol K.
Eo at 298 K. The universal gas constant, R, is 8.314 J/mol K.
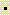 K.
K.
 Ni(CO)4(g)
Ni(CO)4(g) Ho for the reaction be labeled
Ho for the reaction be labeled  Hfo?
Hfo?
 Al(OH)3(s)
Al(OH)3(s)
 POCl3(g)
POCl3(g)
 NO(g)
NO(g)
 CaSO3(s)
CaSO3(s)
 Ho for all these reactions would be labeled
Ho for all these reactions would be labeled  Hfo.
Hfo.
 Ho for the reaction:
Ho for the reaction:
 Na2SO4(g)
Na2SO4(g) Ho
Ho NaOH(s) + 1/2 H2(g)
NaOH(s) + 1/2 H2(g) 2NaOH(s) + SO3(g)
2NaOH(s) + SO3(g) 4Na(s) + 2H2O(l)
4Na(s) + 2H2O(l) Horxn for the following reaction at 25.0 oC:
Horxn for the following reaction at 25.0 oC:

 Hfo (kJ/mol)
Hfo (kJ/mol) Hfo, for FeS2(s), given the following information:
Hfo, for FeS2(s), given the following information:
 2FeO(s) + 4SO2(g)
2FeO(s) + 4SO2(g) Horxn = -1370 kJ
Horxn = -1370 kJ
 Hfo for SO2(g) = -297 kJ/mol
Hfo for SO2(g) = -297 kJ/mol
 Hfo for FeO(s) = -268 kJ/mol
Hfo for FeO(s) = -268 kJ/mol 2BrF3(g)
2BrF3(g)
 Go for the reaction given the following information:
Go for the reaction given the following information:
 2SO3(g)
2SO3(g) Gfo for SO2(g) = -300.4 kJ/mol
Gfo for SO2(g) = -300.4 kJ/mol
 Gfo for SO3(g) = -370.4 kJ/mol
Gfo for SO3(g) = -370.4 kJ/mol
 Ho = +115 kJ and
Ho = +115 kJ and  So = +125 J/K. Calculate
So = +125 J/K. Calculate  Go for the reaction at 25o.
Go for the reaction at 25o.
 S(g) + 2Br2(l)
S(g) + 2Br2(l) G at equilibrium?)
G at equilibrium?)
 H = 30.9 kJ;
H = 30.9 kJ;  S = 93.0 J/K).
S = 93.0 J/K).
 Br2(g)
Br2(g) C,
C,  Ho = +30 kJ;
Ho = +30 kJ;  So = +50 J/K.
So = +50 J/K. Ho for the reaction is +624.7 kJ.
Ho for the reaction is +624.7 kJ.
 SiC(s) + 2CO(g)
SiC(s) + 2CO(g)  H =
H =  E + P
E + P V
V
 H is negative.
H is negative.
 E is negative.
E is negative.
 H.
H.
 H is sometimes exactly equal to
H is sometimes exactly equal to  E.
E.
 H is often nearly equal to
H is often nearly equal to  E.
E.
 H is equal to
H is equal to  E for the reaction:
E for the reaction:
 2H2O(g)
2H2O(g)
 Click here to return to the top.
Click here to return to the top.
 Choose your next chapter:
Choose your next chapter: 
 To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.
To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.