 | Aqueous Equilibrium - Buffers & Titrations
Examples of
Multiple Choice Questions
|
- 1.
- Which of the following combinations cannot produce a buffer solution?
- (a) HNO2 and NaNO2
- (b) HCN and NaCN
- (c) HClO4 and NaClO4
- (d) NH3 and (NH4)2SO4
- (e) NH3 and NH4Br
- 2.
- What is the pH of a solution composed of 0.20 M NH3 and 0.15 M NH4Cl?
- (a) 2.15
- (b) 4.62
- (c) 8.26
- (d) 9.38
- (e) 8.89
- 3.
- Calculate the ratio [CH3COOH]/[NaCH3COO] that gives a solution with pH = 5.00?
- (a) 0.28
- (b) 0.36
- (c) 0.44
- (d) 0.56
- (e) 0.63
- 4.
- Consider a solution which is 0.10 M in CH3COOH and 0.20 M in NaCH3COO. Which of the following statements is true?
- (a) If a small amount of NaOH is added, the pH decreases very slightly.
- (b) If NaOH is added, the OH- ions react with the CH3COO- ions.
- (c) If a small amount of HCl is added, the pH decreases very slightly.
- (d) If HCl is added, the H+ ions react with CH3COOH ions.
- (e) If more CH3COOH is added, the pH increases.
- 5.
- A
buffer was prepared by mixing 1.00 mole of ammonia and 1.00 mole of
ammonium chloride to form an aqueous solution with a total volume of
1.00 liter. To 500 mL of this solution was added 30.0 mL of 1.00 M
NaOH. What is the pH of this solution?
- (a) 8.96
- (b) 9.83
- (c) 9.31
- (d) 9.11
- (e) 9.57
- 6.
- How many grams of NaF would have to be added to 2.00 L of 0.100 M HF to yield a solution with a pH = 4.00?
- (a) 300 g
- (b) 36 g
- (c) 0.84 g
- (d) 6.9 g
- (e) 60. g
- 7.
- Calculate the pH that results when the following solutions are mixed.
- (1) 35 mL of 0.20 M formic acid
- (2) 55 mL of 0.10 M sodium formate
- (3) 110 mL of water
- (a) 3.64
- (b) 3.11
- (c) 4.58
- (d) 3.39
- (e) 4.20
- 8.
- Consider an indicator that ionized as shown below for which its Ka = 1.0 x 10-4
| HIn | + | H2O |  | H3O+ | + | In- |
| yellow | | | | | | red |
Which of the responses contains all the true statements and no others?
- (1) The predominant color in its acid range is yellow.
- (2) In the middle of the pH range of its color change a solution containing the indicator will probably be orange.
- (3) At pH = 7.00, a solution containing this indicator (and no
other colored species) will be red. (Hint: Write the equilibrium
constant expression for the indicator.)
- (4) At pH = 7.00, most of the indicator is in the un-ionized form.
- (5) The pH at which the indicator changes color is pH = 4.
- (a) 1, 3, 5
- (b) 2, 4
- (c) 3, 4, 5
- (d) 1, 2, 3, 5
- (e) another combination
- 9.
- Calculate the pH of the solution resulting from the addition of 20.0 mL of 0.100 M NaOH to 30.0 mL of 0.100 M HNO3.
- (a) 1.35
- (b) 1.70
- (c) 1.95
- (d) 2.52
- (e) 2.80
- 10.
- Which indicator (identified by a letter) could be used to titrate aqueous NH3 with HCl solution?
| Indicator | Acid Range Color | Color-Change pH | Basic Range Color |
| (a) | pink | 1.2 - 2.8 | yellow |
| (b) | blue | 3.4 - 4.6 | yellow |
| (c) | yellow | 6.5 - 7.8 | purple |
| (d) | colorless | 8.3 - 9.9 | red |
| (e) | none of these | | |
- 11.
- Consider
the titrations of the pairs of aqueous acids and bases listed on the
left. For which pair is the pH at the equivalence point stated incorrectly?
| Acid-Base Pair | pH at Equivalence Point |
| (a) HCl + NH3 | less than 7 |
| (b) HNO3 + Ca(OH)2 | equal to 7 |
| (c) HClO4 + NaOH | equal to 7 |
| (d) HClO + NaOH | less than 7 |
| (e) CH3COOH + KOH | greater than 7 |
- 12.
- What is the pH at the equivalence point in the titration of 100.0 mL of 0.20 M ammonia with 0.10 M hydrochloric acid?
- (a) 4.6
- (b) 5.2
- (c) 7.0
- (d) 5.5
- (e) 4.9
- 13.
- Calculate the pH of a solution prepared by mixing 300 mL of 0.10 M HF and 200 mL of 0.10 M KOH.
- (a) 2.82
- (b) 2.96
- (c) 3.32
- (d) 3.44
- (e) 3.53
- 14.
- What is the approximate pH of a solution prepared by mixing equal volumes of 0.05 M methylamine and 0.20 M hydrochloric acid?
- (a) 2.57
- (b) 1.12
- (c) 1.63
- (d) 10.5
- (e) 9.8
- 15.
- Which of the following salts give acidic aqueous solutions?
| (1) KNO3 | (2) KCH3COO | (3) NH4NO3 | (4) RbI |
| (5) (NH4)2SO4 | (6) BaCl2 | (7) NaCN | (8) KNO2 |
- (a) 2, 7, 8
- (b) 3, 5
- (c) 2, 4, 6
- (d) 1, 4, 7, 8
- (e) 1, 4, 6
- 16.
- The following titration curve is the kind of curve expected for the titration of a ____ acid with a ____ base.
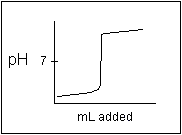
- (a) strong, strong
- (b) weak, strong
- (c) strong, weak
- (d) weak, weak
- (e) none of these
- 17.
- Consider
the titration of 30.0 mL of 0.20 M nitrous acid by adding 0.0500 M
aqueous ammonia to it. The pH at the equivalence point is _____. (Note:
This is the titration of a weak acid with a weak base.)
- (a) greater than 7
- (b) equal to 7
- (c) less than 7
- (d) cannot be determined without more data (not including Ka and Kb)
- (e) is impossible to predict
Answers:
1. (c) 2. (d) 3. (d) 4. (c) 5. (c) 6. (e) 7. (a) 8. (d) 9. (b) 10. (b) 11. (d) 12. (b) 13. (d) 14. (b) 15. (b) 16. (a) 17. (c)

 Click here to return to the top.
Click here to return to the top.

 Choose your next chapter:
Choose your next chapter:
Fundamentals of Chemistry
| Chemical Formulas & Composition Stoichiometry
| Chemical Equations & Rxn Stoichiometry
| Types of Chemical Reactions
|
| Atomic Structure
| Chemical Periodicity
| Chemical Bonding
| Molecular Structure/Covalent Bonding Theories| Molecular Orbital Theory |
| Acids/Bases/Salts - Theory & Rxns
| Acids/Bases/Salts - Calculations (including balancing redox rxns)
| Gases
| Solids & Liquids
| Solutions |
| Thermodynamics
| Kinetics
| Equilibrium
| Aqueous Equilibrium - Acids/Bases/Salts
| Aqueous Equilibrium - Buffers & Titrations
|
| Aqueous Equilibrium - Slightly Soluble Salts
| Electrochemistry
| Metallurgy
| Metal Properties & Rxns
| Nonmetals & Metalloids
|
| Coordination Compounds
| Nuclear Chemistry
| Organic Chem - Formulas/Names/Properties
| Organic Chem - Shapes/Rxns/Biopolymers |

 To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.
To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.





 Click here to return to the top.
Click here to return to the top.
 Choose your next chapter:
Choose your next chapter: 
 To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.
To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.