 | Chemical Formulas and Composition Stoichiometry
Examples of
Multiple Choice Questions
|
- 1.
- The formula weight of the compound, Al2(SO4)3
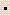 18H2O is:
18H2O is:
- (a) 394.4 g
- (b) 666.4 g
- (c) 110,900 g
- (d) 466.8 g
- (e) 561.2 g
- 2.
- The weight of a millimole of (NH4)2HPO4 is:
- (a) 132 g
- (b) 114 g
- (c) 1.14 x 10-3 g
- (d) 0.132 g
- (e) 6.02 x 1020 g
- 3.
- How many moles of alanine, C3H7NO2, are there in 159 g of alanine?
- (a) 1.42 x 104
- (b) 1.78
- (c) 0.992
- (d) 0.560
- (e) 3.31
- 4.
- How many atoms are in one mole of CH3OH?
- (a) 6
- (b) 6.0 x 1023
- (c) 12.0 x 1023
- (d) 3.6 x 1024
- (e) 3
- 5.
- The mass in grams of 2.6 x 1022 chlorine atoms is:
- (a) 4.4
- (b) 11
- (c) 0.76
- (d) 1.5
- (e) 3.2
- 6.
- How many aluminum atoms are there in 3.50 grams of Al2O3?
- (a) 4.13 x 1022
- (b) 4.90 x 1022
- (c) 2.07 x 1022
- (d) 1.68 x 1022
- (e) 2.45 x 1022
- 7.
- Which one of the samples contains the most atoms?
- (a) 1 mol of CO2(g)
- (b) 1 mol of UF6(g)
- (c) 1 mol of CH3COCH3(l)
- (d) 1 mol of He(g)
- (e) all contain the same number of atoms
- 8.
- Which one of the samples contains the most molecules?
- (a) 1 mol of CO2(g)
- (b) 1 mol of UF6(g)
- (c) 1 mol of CH3COCH3(l)
- (d) 1 mol of He(g)
- (e) all contain the same number of molecules
- 9.
- Which one of the samples has the largest mass?
- (a) 1 mol of CO2(g)
- (b) 1 mol of UF6(g)
- (c) 1 mol of CH3COCH3(l)
- (d) 1 mol of He(g)
- (e) all have the same mass
- 10.
- Which of the following statements is(are) FALSE?
- 1. The percent by mass of each element in a compound depends on the amount of the compound.
- 2. The mass of each element in a compound depends on the amount of the compound.
- 3. The percent by mass of each element in a compound depends on the amount of element present in the compound.
- (a) 2 and 3
- (b) 1 only
- (c) 1 and 2
- (d) 1, 2 and 3
- (e) another combination
- 11.
- Guanidin, HNC(NH2)2, is a fertilizer. To three significant figures, what is the percent by mass of nitrogen in the fertilizer?
- (a) 45.2%
- (b) 49.4%
- (c) 54.8%
- (d) 65.1%
- (e) 71.1%
- 12.
- Calculate the percent, by weight, of carbon in 154 g of C4H8O3?
- (a) 46%
- (b) 31%
- (c) 72%
- (d) 27%
- (e) 55%
- 13.
- Analysis
of a sample of a covalent compound showed that it contained 14.4%
hydrogen and 85.6% carbon by mass. What is the empirical formula for
the compound?
- (a) CH
- (b) CH2
- (c) CH3
- (d) C2H3
- (e) none of these
- 14.
- An oxide of lead contains 90.65% Pb, by weight. The empirical formula is:
- (a) Pb
- (b) PbO
- (c) Pb3O4
- (d) Pb2O3
- (e) PbO2
- 15.
- A
0.500 g sample of a compound containing only antimony and oxygen was
found to contain 0.418 g of antimony and 0.082 g of oxygen. What is the
simplest formula for the compound?
- (a) SbO
- (b) SbO2
- (c) Sb3O4
- (d) Sb2O5
- (e) Sb2O3
- 16.
- A
compound contains, by mass, 40.0% carbon, 6.71% hydrogen, and 53.3%
oxygen. A 0.320 mole sample of this compound weighs 28.8 g. The
molecular formula of this compound is:
- (a) C2H4O2
- (b) C3H6O3
- (c) C2H4O
- (d) CH2O
- (e) C4H7O2
- 17.
- What mass of cerussite, PbCO3, would contain 35.0 grams of lead?
- (a) 27.1 g
- (b) 45.1 g
- (c) 42.4 g
- (d) 35.6 g
- (e) 51.7 g
Answers:
1. (b) 2. (d) 3. (b) 4. (d) 5. (d) 6. (a) 7. (c) 8. (e) 9. (b) 10. (b) 11. (e) 12. (a) 13. (b) 14. (c) 15. (e) 16. (b) 17. (b)

 Click here to return to the top.
Click here to return to the top.

 Choose your next chapter:
Choose your next chapter:
Fundamentals of Chemistry
| Chemical Formulas & Composition Stoichiometry
| Chemical Equations & Rxn Stoichiometry
| Types of Chemical Reactions
|
| Atomic Structure
| Chemical Periodicity
| Chemical Bonding
| Molecular Structure/Covalent Bonding Theories| Molecular Orbital Theory |
| Acids/Bases/Salts - Theory & Rxns
| Acids/Bases/Salts - Calculations (including balancing redox rxns)
| Gases
| Solids & Liquids
| Solutions |
| Thermodynamics
| Kinetics
| Equilibrium
| Aqueous Equilibrium - Acids/Bases/Salts
| Aqueous Equilibrium - Buffers & Titrations
|
| Aqueous Equilibrium - Slightly Soluble Salts
| Electrochemistry
| Metallurgy
| Metal Properties & Rxns
| Nonmetals & Metalloids
|
| Coordination Compounds
| Nuclear Chemistry
| Organic Chem - Formulas/Names/Properties
| Organic Chem - Shapes/Rxns/Biopolymers |

 To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.
To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.


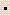 18H2O is:
18H2O is:

 Click here to return to the top.
Click here to return to the top.
 Choose your next chapter:
Choose your next chapter: 
 To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.
To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.