|


Department of Chemistry Texas A&M University PO Box 30012 College Station, TX 77842-3012 (t) 979.845.0215 (l) 979.845.4732 (f) 979.847.8660 |
Excision Versus Synthesis of Molybdenum
Chalcogenide Clusters
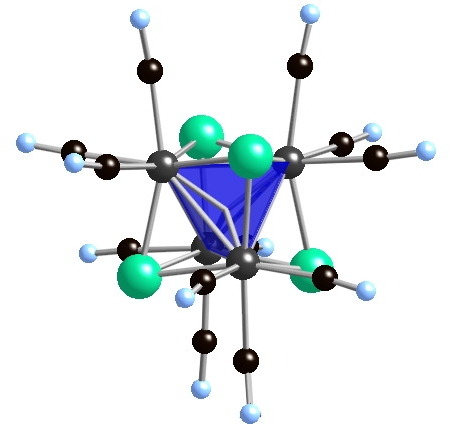
The 'Chevrel Phases', 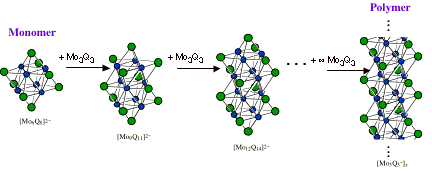
We and others are interested in isolating and derivatizing discrete cluster subunits of these solids, so that these electroactive clusters can be studies as isolated species and perhaps exploited in the preparation of either novel conducting materials or liquid crystals.
Preparative work aimed at the formation of Mo6Se8 clusters has involved the assembly from smaller cluster fragments, Mo3Q4, or the Q/Cl exchange starting with a [Mo6Cl8]4+ cluster. Fedorov and coworkers recently discovered that the binary Chevrel phase compound Mo6Se8 serves as a precursor to discrete [Mo6Se8(CN)6]7- and [Mo6Se8(CN)6]6- cluster ions when reacted with molten KCN. Though the reactivity and ligand field strength of cyanide makes it an attractive nucleophile for such a ³cluster excision² process, it seemed remarkable that the Mo6Se8 clusters might be extricated intact from the tightly crosslinked Chevrel phase structure.
These reports suggest the potential use of cyanides as a reaction media for excising or synthesizing chalcogenide clusters. Molten cyanides may serve as a medium for excision or synthesis of other, larger molybdenum chalcogenide clusters. One focus of this research is the use of metal cyanides as a medium for isolating new molybdenum chalcogenide cluster species. Specifically:
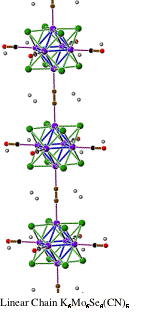
The reaction of molten KCN with Mo6Se8 under anaerobic conditions, yields the new linear chain compound, K6[Mo6Se8(CN)4(CN)2/2]. This one-dimensional chain compound, K6[Mo6Se8(CN)4(CN)2/2] crystallizes in a body-centered tetragonal structure (I4/m, a = 11.50585(4) Å, c = 9.5177(4) Å), shown in Figure 3. Each of the four molybdenum atoms in the basal-plane of each [Mo6Se8]- cluster are bound to a terminal CN- ligand, whereas each apical molybdenum atom is bound to a bridging cyanide that links adjacent [Mo6Se8]- clusters to form linear chains, [Mo6Se8(CN)4(CN)2/26-]n, that propagate up the c-axis.
Theoretical treatments for [Mo6Se8]- core indicate that there are 21 cluster bonding electrons and an eg1 electron configuration (2Eg state) is expected. In the observed tetragonal environment (nominally D4h), the eg (Oh) orbital set will split such that the a1g(D4h) descendent will be the SOMO (singly occupied MO) and the b1g(D4h) orbital will be unoccupied. The EPR spectrum (T = 10K) is characteristic of an axial system; averaging of g|| (1.9822(1)) and g^ (2.4425(1)) gives g-value of 2.289(1) (Figure 4c). Over the temperature range 1.8 400K, magnetic susceptibility data are well described by the Curie-Weiss relation, c = c0 + C/(T + q); c0 = 1.216 ´ 10-5 emu/mol, C = 0.497 emu·K/mol, and q = 2.74 K. Magnetic ordering was not observed at any temperature. The magnetic moment obtained from the Curie constant C is 1.99 B.M. (meff = 2.828 C1/2). The magnetic data shows that the unpaired cluster electrons are uncoupled by the bridging cyanide ligands.
|
|
(Updated: 8/30/2002) |
E-Mail Professor Hughbanks: trh@mail.chem.tamu.edu |


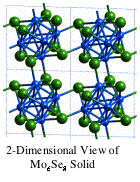 MxMo6Q8
(Q = S, Se, Te) came to the forefront of solid-state chemistry in the 1970s and
1980s. These molybdenum chalcogenides attracted intense interest because of
their diverse physical and chemical properties, such as high-critical field (H
MxMo6Q8
(Q = S, Se, Te) came to the forefront of solid-state chemistry in the 1970s and
1980s. These molybdenum chalcogenides attracted intense interest because of
their diverse physical and chemical properties, such as high-critical field (H