

Our research considers fundamental questions of optical energy conversion relating to plasmonic and inorganic nanoscale materials. Our experiments are principally designed to identify and optimize unique nanoscale phenomena useful for solar energy conversion, as well as related opportunities at the intersection of nanophotonics and chemistry for broad application beyond the scope of solar energy.
The current world record solar cell operates at 44.4% power conversion efficiency. Thermodynamic analyses indicate that much higher efficiency is theoretically possible. Indeed, technical challenges, rather than laws of nature, limit current solar power convertors from achieving the maximum thermodynamic efficiency of 95%.
Shockley and Queisser pointed out that the maximum efficiency for a single junction photovoltaic solar cell is 33%. Their calculation is based on several assumptions about the microscopic process of absorption and re-emission of radiation by the solar cell. We explore how optical nanomaterials may modify these underlying assumptions to provide opportunities for achieving even higher conversion efficiencies.
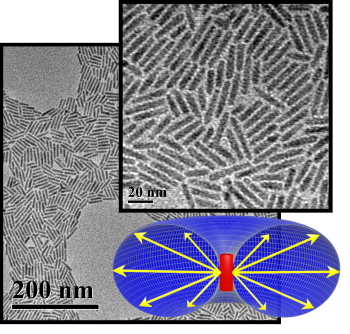
Semiconductor nanorods have the unique ability to rectify light into a solar cell by changing the angles of light entering or leaving the device. We aim to define the maximum benefits provided by this effect.
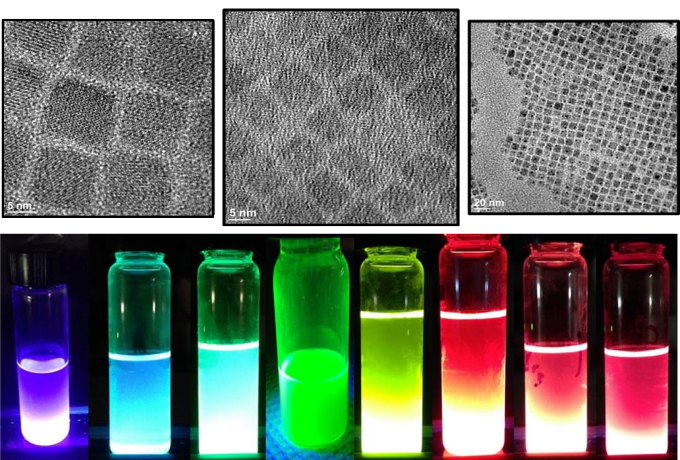
We are interested in using more complex doped or heterogeneous, highly fluorescent nanoparticles to promote luminescent up-conversion – that is, to absorb two low energy photons and emit one higher energy photon.
Fundamentally plasmon oscillations are an opto-mechanical phenomenon. Incident optical fields induce the coherent motion of electrons that produce large fluctuations of charge density at ‘hot spots’ in resonant structures. We are interested to know if this microscopic electronic motion can provide useful electrical or chemical work directly – without the use of semiconductors or other non-plasmonic structural elements. Our ultimate vision is all-metal energy converters, such as solar cells or photo-chemical catalysts.
Unlike conventional thermionic emission, where metal cathode emitters should be brought to high temperatures (~1500K), a fraction of the electronsin plasmonic metal nanostructures can maintain temperatures which greatly exceed that of the lattice during steady-state illumination. Due to the approximately 100 times smaller heat capacity of electron gas in the metals,these hot electrons can be thermally emitted into a vacuum and then collected. We are interested in understanding and optimizing the dependence on the incident light wavelength and power, the size and shape of the nanostructures, and the device design to produce this thermionic current.
Hot electrons can be generated through the decay of plasmons and can achieve temperatures that far exceed their surroundings. Using quantitative spectroscopic analysis based on continuous wave electronic Raman spectroscopy, we have been able to separate spectral contributions of different electronic populations, allowing us to identify the temperatures and populations of hot electrons. Using this information, we can identify the hot electron contribution during chemical reaction catalysis. For example, we areinterested in knowing the hot electron conditions required for the possible reduction of atmospheric carbon dioxide to amorphous carbon.
The Inverse Faraday Effect (IFE) is an optomagnetic phenomenon that manifests as an induced magnetization when a material is illuminated by circularly polarized light. Previously, we were able to confirm the existence ofmagnetic fields due to the IFE in plasmonic gold nanoparticles by performing ultrafast pump-probe measurements. Currently we are using Raman thermometry methods developed in our group to probe the differences of temperature in plasmonic nanostructures when illuminated by linearly or circularly polarized light. This can help us better understand the mechanism of IFE in plasmonic materials.
When the resonance of an optical cavity is tuned to the same energy of a molecular vibrational mode, the two systems can coherently exchange energy. If the energy exchange occurs faster than the losses of the system, new hybrid “polariton” modes are formed with higher and lower energies than the original resonance, which can subsequently modify of the bond energy of the molecule. This is otherwise known as vibrational strong coupling. We aim to leverage advances in the field of plasmonic nanomaterials to strongly couple films of molecules to a metal substrate for modified chemistry, such as reaction rates and site-selective chemical catalysis.