Disulfide Bond Elucidation using Capillary Electrophoresis – Mass Spectrometry
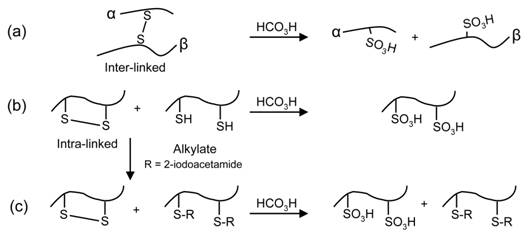
Disulfide bonds play important roles in establishing and stabilizing three-dimensional protein structure and mass spectrometry (MS) has become the primary detection method to decipher their biological and pathological roles. Several experimental methods before or after MS detection have been developed to aid in disulfide bond assignment, such as tandem MS followed by database searching or modification of the disulfide bond via chemical reduction or oxidation. Despite these technological advancements, the detection and proper assignment of disulfide bonds has remained experimentally difficult. Therefore, we have developed an alternative method for disulfide bond elucidation using capillary electrophoresis – mass spectrometry (CE-MS) combined with an on-target performic acid oxidation method for matrix assisted laser desorption/ionization (MALDI) deposited samples. The on-target oxidation method offers (i) no post-oxidation sample cleanup, (ii) improved throughput over solution-phase oxidation methods, and (iii) can easily be adapted to CE separations coupled off-line with MALDI-MS. The schematic above illustrates the resulting performic acid oxidation products for both (a) inter-linked and (b) intra-linked disulfide-containing peptides.1 Currently, we are investigating the effect of the cysteic acid position on the resulting fragment ion types observed in the negative ion fragmentation mass spectra.
|
 LABORATORY FOR BIOLOGICAL
LABORATORY FOR BIOLOGICAL