Laganowsky Research Laboratory
Background
Innovation
Kir Channels
K2P Channels
ABC Transporters
MAPK Pathway
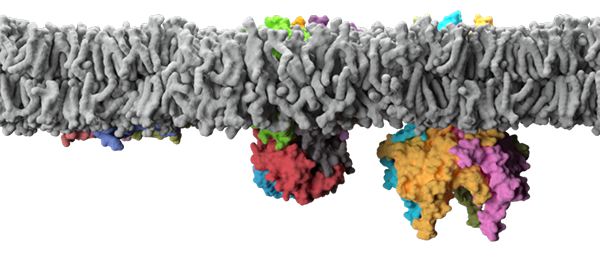
Membrane proteins are embedded in the biological membrane where they intimately interact with lipids. They represent one of the most important targets for pharmaceutical drug discovery, with a staggering 60% of drugs on the market targeting integral membrane proteins. The membrane environment is composed of a rich repertoire of chemically diverse lipids, with over 40,000 biologically relevant structures in the LIPID MAPS Structure Database to date. This striking number is over seven-fold more than the predicted human membrane proteome! Moreover, the lipid environment is dynamic, for example bacteria, in response to temperature, alter the degree of saturation and acyl length of their lipids to adjust membrane fluidity. In the case of eukaryotic cells, lipids are distributed and maintained asymmetrically among the leaflets of organelles. Lipids are also highly organized within membranes forming distinct domains, for example caveolae, and micro domains, for example lipid rafts, that are enriched in specific lipids with unique functional properties. Despite the growing realization of the complexity of the biological membrane, we actually know little about how lipid molecules influence the structure and function of membrane proteins on the molecular level.
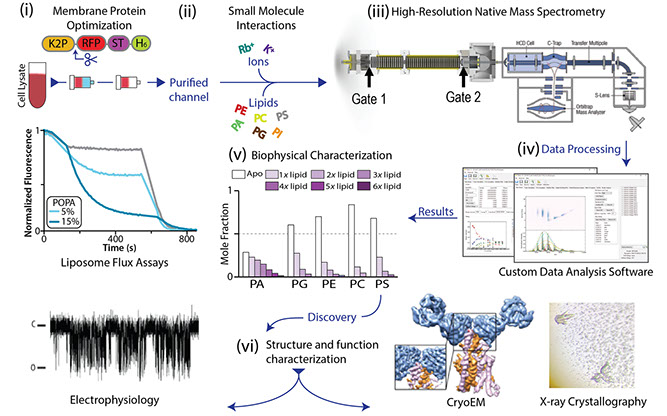
The crucial role of lipids in the folding, structure, and function of membrane proteins is emerging from exceedingly more reports. However, a long-standing problem in this area of membrane protein biology has been the lack of technology to investigate membrane protein-lipid interactions. Of late, my laboratory has been developing new and innovative approaches to interrogate membrane protein-lipid interactions down to the resolution of individual lipid binding events using native ion mobility mass spectrometry (IM-MS), a technique where non-covalent interactions are preserved in the mass spectrometer. Recently, we have developed cutting-edge approaches to obtain for the first time thermodynamic binding parameters for individual lipid binding events to membrane proteins and the ability to interrogate heterogeneous lipid binding events to membrane proteins at the resolution of individual lipid molecules. In short, we are developing new and innovative native ion mobility mass spectrometry methods to address key questions in the field that remain otherwise intractable using other biophysical methods.
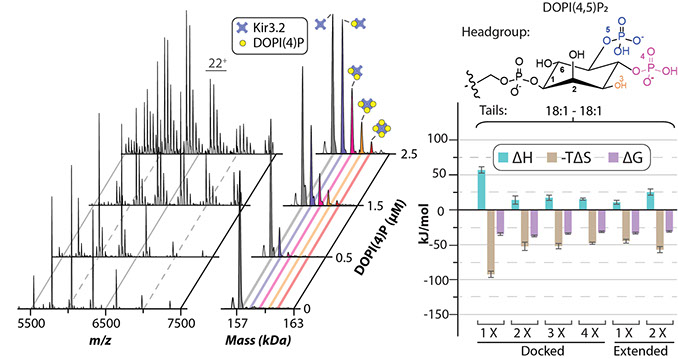
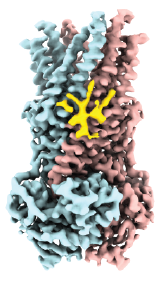
Inward rectifying potassium (Kir) channels have essential roles in a number of physiological processes and are known to require a specific lipid for function. Kir channels are expressed in tissues throughout the body and genetic alterations in these channels have been linked to a number of diseases. Kir channels are primarily located in the plasma membrane, where they regulate membrane potential and potassium homeostasis, and particular mutations in these channels result in improper trafficking that are linked to Andersen syndrome. In particular, phosphatidylinositol 4,5-bisphosphate (PIP2), a minor component of the cytoplasmic leaflet, is required for activation of all Kir channels. Most interestingly, mutations in residues important for binding PIP2 are associated with Bartter and Andersen syndromes and other diseases. Kir channels are also regulated by a number of other molecules including phosphorylation by kinases A and C, sodium, pore blockers (polyamine, Mg2+), and ethanol. There are seven subfamilies of Kir channels: classical Kir channels (Kir2.x) are strong rectifiers that have central roles in cardiac inward rectifier current; Kir3.x channels are unique in that they require G proteins in addition to PIP2 for function with some of these channels modulated by sodium ions; ATP-sensitive potassium channels (Kir6.x); and transport potassium channels (Kir1.x, Kir4.x, Kir5.x, and Kir7.x).
The TREK subfamily of two-pore domain potassium channels (K2P2.1/TREK-1, K2P10.1/TREK-2, and K2P4.1/TRAAK) are responsible for background K+ currents and implicated in a number of physiological processes, such as pain perception; dysfunction of K2P channels are associated with developmental and neurological disorders, such as epilepsy and intellectual disability. The TREK subfamily are predominantly expressed throughout the entire nervous system and have emerged as attractive therapeutic targets in depression, memory disorders, and pain. The channels are also modulated by diverse stimuli including anesthetics and natural effectors such as lipids, temperature, and pressure. Over the past decade, crystal structures for all members of this subfamily have provided insight into channel gating mechanisms and enabled visualization of small molecule binding, including sites for activators, inhibitors and lipids. However, the identity and role of the bound lipids remain largely unknown, representing a significant void in our understanding. It also remains poorly understood if lipid binding events to the TREK subfamily can alter remote binding sites for small molecules.

The transport of various biological chemicals, such as lipids, ions, vitamins, steroids, toxins and antibiotics, across the plasma membrane of cells is regulated by members of the ATP-Binding Cassette (ABC) transporter superfamily. These dimeric, membrane-embedded proteins are typically comprised of a transmembrane region (5-10 α-helices) and two solvent-exposed, highly conserved, nucleotide-binding domains (NBDs). These NBDs are the active sites of ATP hydrolysis, which produces ADP and an inorganic phosphate (ATP → ADP + Pi), and power the transport of various molecules either in or out of the cell via the highly variable, although often highly specific, transmembrane domain. This vital transportation process of the cell requires the ABC transporter to be highly dynamic, with some reports of the NBDs displacing up to 10 nm upon ATP hydrolysis.
One of the most well-studied ABC transporters is MsbA from E. coli that is involved in the transport of the lipopolysaccharide (LPS) core from the cytosolic to the periplasmic leaflet of the inner membrane. LPS is the major component responsible for an animal immune response to an Enterobacteriae infection; as such, MsbA is a highly attractive drug target as inhibition or deletion of this gene is lethal.
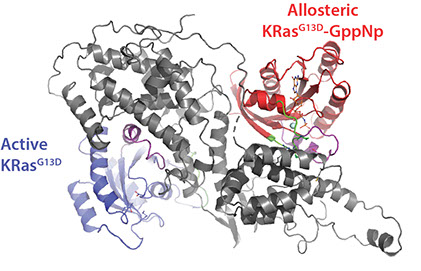
The RAS-RAF-MEK-ERK (or MAPK) signaling pathway is involved in cell proliferation, differentiation, survival and senescence. The RAS proteins (KRAS, HRAS, and NRAS) act as a molecular switch by cycling between active (GTP-bound) and inactive (GDP-bound) forms that couple extracellular and intracellular signaling networks. As RAS proteins possess low guanidine nucleotide exchange rates, they are regulated by a specific guanine nucleotide exchange factor Son of Sevenless (SOS), which activates RAS by facilitating the exchange of GDP-bound RAS with GTP. The catalytic activity of SOS is also allosterically modulated by an active, GTP-bound RAS. Importantly, disrupting the interaction between RAS and SOS has emerged as an attractive therapeutic strategy. Despite decades of research on RAS, the molecular assemblies formed between wild-type and oncogenic RAS mutants remains poorly understood. Moreover, RAS activates RAF kinases (ARAF, BRAF, and CRAF), a protein kinase that is also mutated in some human cancers. Recent structures of BRAF complexes have shed light on the assemblies. However, the complexes RAF forms with RAS and other molecules, such as copper, remains unclear. Therefore, there is a critical need to apply new technology to better understand the molecular complexes formed between oncogenic RAS mutants and other proteins.
Department of Chemistry, 301 Old Main Dr, ILSB RM 1165, College Station, Texas, 77845