Mechanisms of Catalysis in Polymers and CO2 Reduction
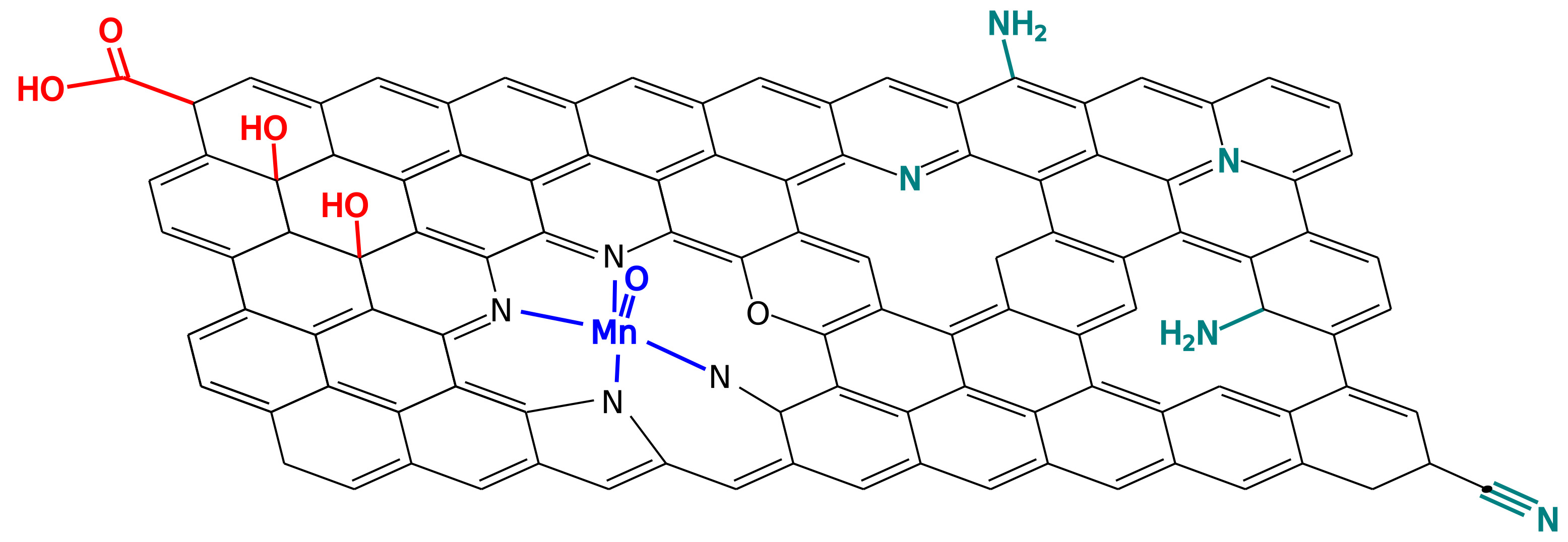
Materials containing dispersed sites with well defined chemical structures are promising to be used in the design of new classes of catalysts. The structural basis for the mechanisms and activity of many such heterogeneous single site catalysts however still remain unknown. We are elucidating the relationship between active site structures and catalytic mechanisms for catalysts based on graphene. These catalysts have an application in
CO2 reduction, a reaction that has been proposed for environmental carbon capturing.
Widely applied
polymer materials are synthesized by chemical catalysts, such as organometallic complexes. Knowing the relationship between synthesis and the properties of the final product is required for the design of such reactions. The kinetics and mechanisms of the catalyzed reactions are governed by the interaction of the monomer and catalyst, and determining the structure of the final product. We are characterizing the activity, deactivation pathways, stereo structure formation, and other properties of modern polymerization catalysts.
Hyperpolarization provides the signal sensitivity enabling to characterize reactions by fast
13C NMR spectroscopy, when reactant and product distributions are far from equilibrium. Product structures and reaction kinetics are identified in real-time through obervation of chemical shifts and of polarization transfer throughout the reaction. Interatomic distances in active sites can be measured by moedling of observed relaxation properties. Altogether, the experimental determination of structure and kinetics contributes to the fundamental understanding of the mechanisms of reactions and will assist in the design of new complexes and materials serving as catalysts.
Recent Publication
Kim, Y., Samouei, H. and Hilty, C. Polyolefin
Catalysis of Propene, 1-Butene and Isobutene Monitored Using Hyperpolarized NMR. Chem. Sci. 12(8): 2823-2828 (2021).
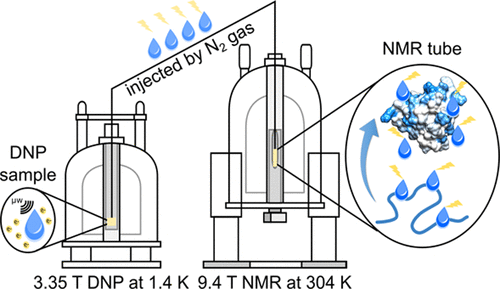 Nuclear spin hyperpolarization provides new modalities for biomolecular NMR, to investigate the structure, dynamics and interactions of biological macromolecules. We are using dissolution dynamic nuclear polarization (D-DNP) to produce non-equilibrium spin states that enhance sensitivity by large factors, often of three orders of magnitude or more. Hyperpolarization dramatically reduces limits of detection into the nanomolar concentration range, increases the speed of signal acquisition, and enables label-free selectivity, while retaining the chemical information available from NMR spectroscopy.
Protein-ligand interactions play a pivotal role in fundamental biological processes including cellular signaling and regulation. Experimental screening for interactions with drug candidate compounds and fragments is an indispensable step in drug discovery. Traditional methods for determining such interactions often require highly purified proteins, which in particular are not always available in the case of membrane proteins. Notwithstanding, 60% of current drugs target membrane proteins. We are developing methods for determining interactions between small molecules of arbitrary type, directly with receptors or other components located on the surface or within a cell.
Nuclear spin hyperpolarization provides new modalities for biomolecular NMR, to investigate the structure, dynamics and interactions of biological macromolecules. We are using dissolution dynamic nuclear polarization (D-DNP) to produce non-equilibrium spin states that enhance sensitivity by large factors, often of three orders of magnitude or more. Hyperpolarization dramatically reduces limits of detection into the nanomolar concentration range, increases the speed of signal acquisition, and enables label-free selectivity, while retaining the chemical information available from NMR spectroscopy.
Protein-ligand interactions play a pivotal role in fundamental biological processes including cellular signaling and regulation. Experimental screening for interactions with drug candidate compounds and fragments is an indispensable step in drug discovery. Traditional methods for determining such interactions often require highly purified proteins, which in particular are not always available in the case of membrane proteins. Notwithstanding, 60% of current drugs target membrane proteins. We are developing methods for determining interactions between small molecules of arbitrary type, directly with receptors or other components located on the surface or within a cell.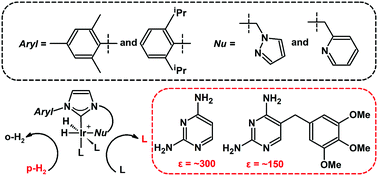 Para-hydrogen, the anti-parallel nuclear spin isomer of the hydrogen molecule, induces large spin polarization when reacted with target molecules, or when interacting by the intermediary of polarization transfer catalysts. Para-hydrogen based NMR is rapid and inexpensive, but challenges remain for applications in the life sciences.
Para-hydrogen, the anti-parallel nuclear spin isomer of the hydrogen molecule, induces large spin polarization when reacted with target molecules, or when interacting by the intermediary of polarization transfer catalysts. Para-hydrogen based NMR is rapid and inexpensive, but challenges remain for applications in the life sciences. Materials containing dispersed sites with well defined chemical structures are promising to be used in the design of new classes of catalysts. The structural basis for the mechanisms and activity of many such heterogeneous single site catalysts however still remain unknown. We are elucidating the relationship between active site structures and catalytic mechanisms for catalysts based on graphene. These catalysts have an application in CO2 reduction, a reaction that has been proposed for environmental carbon capturing.
Materials containing dispersed sites with well defined chemical structures are promising to be used in the design of new classes of catalysts. The structural basis for the mechanisms and activity of many such heterogeneous single site catalysts however still remain unknown. We are elucidating the relationship between active site structures and catalytic mechanisms for catalysts based on graphene. These catalysts have an application in CO2 reduction, a reaction that has been proposed for environmental carbon capturing.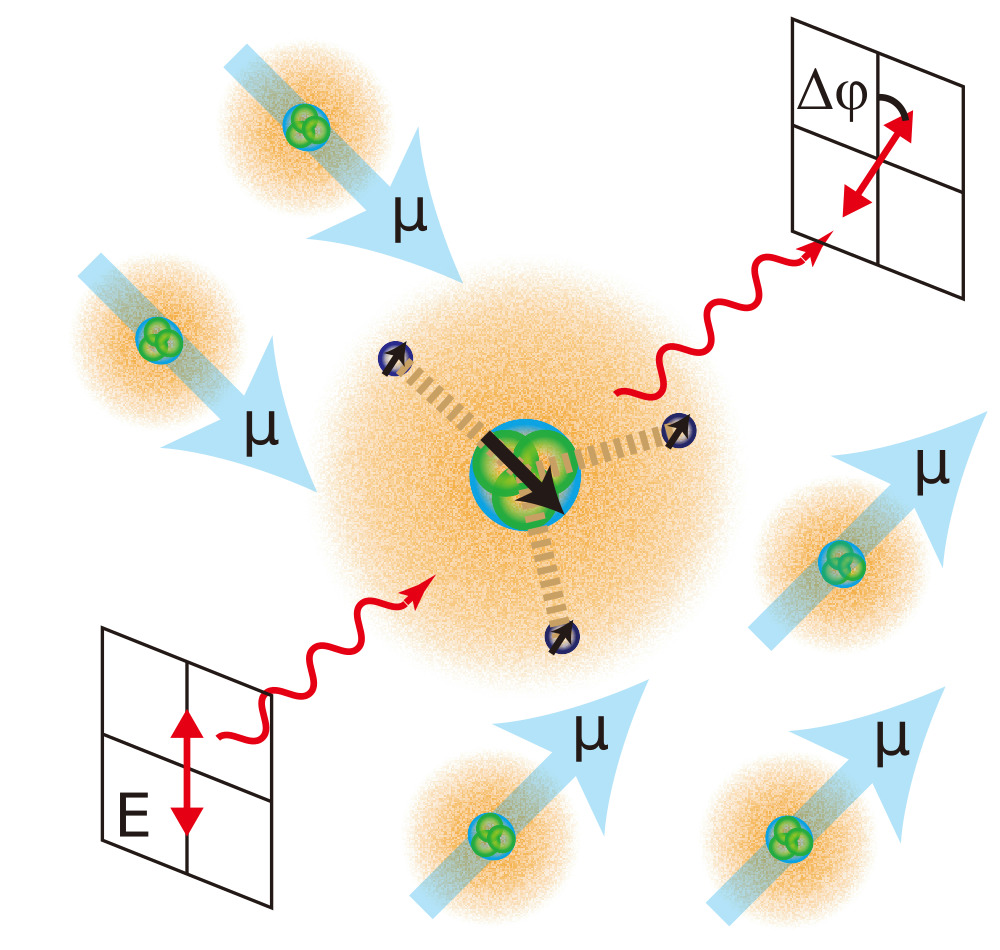 We are developing nuclear-spin optical rotation for enhancing the information content in optical and nuclear magnetic resonance spectroscopy. The technique correlates nuclear spin states and electronic structure through dynamic vector polarizability, thereby providing a route to obtaining localized information on electronic structure. The severe obstacle for meaningful application is that the measured effect is normally extremely small, requiring substantial time for detection. Progress in the application of nuclear-spin optical rotation to chemistry critically depends on strategies for signal enhancement. We are applying dynamic nuclear polarization to generate non-equilibrium nuclear spin states. Hyperpolarized samples can increase the nuclear-spin optical rotation signal 100-100,000 times, depending on the nuclei and experimental conditions and enable application of the method for the characterization of various molecules and materials. The realization of this combined NMR and optical spectroscopy offers a powerful analytical method for fundamental studies of light-matter interaction in liquids and solid materials with future applications in energy harvesting devices and quantum information storage.
We are developing nuclear-spin optical rotation for enhancing the information content in optical and nuclear magnetic resonance spectroscopy. The technique correlates nuclear spin states and electronic structure through dynamic vector polarizability, thereby providing a route to obtaining localized information on electronic structure. The severe obstacle for meaningful application is that the measured effect is normally extremely small, requiring substantial time for detection. Progress in the application of nuclear-spin optical rotation to chemistry critically depends on strategies for signal enhancement. We are applying dynamic nuclear polarization to generate non-equilibrium nuclear spin states. Hyperpolarized samples can increase the nuclear-spin optical rotation signal 100-100,000 times, depending on the nuclei and experimental conditions and enable application of the method for the characterization of various molecules and materials. The realization of this combined NMR and optical spectroscopy offers a powerful analytical method for fundamental studies of light-matter interaction in liquids and solid materials with future applications in energy harvesting devices and quantum information storage.