

Welcome to the application for the NSF Center for Mechanical Control of Chemistry (CMCC) Research Experience for Undergraduates (REU) program, part of the National Science Foundation (NSF) Centers for Chemical Innovation (CCI) program. In this REU program, undergraduate students will carry out fundamental research as a full member of a research group at one of the affiliated center locations. In addition to research, students will participate in professional development and science communication in collaboration with the Science History Institute in Philadelphia, PA.
In mechanochemistry, the application of mechanical force can help drive reactions at lower temperatures and without the use of solvents, making it a green approach to chemical synthesis. A key challenge, however, is the lack of understanding of how precise forces can be applied to chemical systems to obtain specific products.
The cross-cutting nature of mechanochemistry blends chemistry, materials science, engineering, and physics, and thus lends itself to a unique research experience for students. Our diverse team of faculty has a strong record of supporting integrative, interdisciplinary, collaborative research activities, and in fostering an inclusive and supportive climate that welcomes and supports everyone. Participation in our REU program will allow students to gain experience in team-based research and afford them the opportunity for professional development in science communications, innovation, STEM history, and preparing for graduate school. All of these aspects will support and foster collaboration across multiple research disciplines.
Professional Development Highlights:
Students in the CMCC REU will interact across our multi-institutional center via team-based projects and will receive personal mentoring from multiple faculty and graduate students.
Students in our REU Program will receive the following support:
This is a competitive program open to undergraduates in chemistry, physics, materials science, chemical engineering, or mechanical engineering majors (or closely related fields) enrolled in 4-year U.S. colleges and universities who have completed at least their first year with a 3.0 GPA or better. Two letters of recommendation will also be required. Students should not be in their final year of study (i.e. graduating before the REU program begins). Students must be US citizens or permanent residents.
To complete the application on the next page, you will need to provide:
The following research topics are available to REU students in this program:
Participating Sites: TAMU (Experimental); UC Merced and UPenn (Computational)
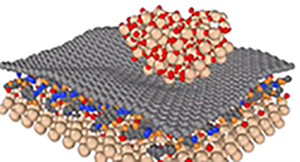
To foster our understanding of how the precise application of mechanical force influences reactions, in this theme, students will investigate reactions on 2D nanomaterials, such as graphene, transition metal dichalcogenides and MXenes, and explore force-inducted transitions in novel layered materials like indium bismuth (InBi), and layered lanthanides, such as neodymium iodine (NdOI). Detailed knowledge of the positions of the atoms, coupled with applying forces with techniques such as atomic force microscopy, allow us to study how applied forces influence, for example, radial and pericyclic additions on surfaces, or bond breaking and reforming on surfaces and between layers of 2D materials. Combined with density functional theory (DFT) and molecular dynamics (MD) simulations, a holistic picture of how force drives reactions on 2D interfaces is revealed, allowing students to gain experience in both experimental and theoretical aspects of mechanochemistry.
Participating Sites: CUNY, TAMU and UPenn (Experimental); CUNY (Computational)
Substitution and elimination reactions are the most common chemical transformations and have an important role in the fabrication of pharmaceuticals and advanced materials, however, solvent waste, poor-selectivity, and low-yields associated with these reaction result in a high-environmental cost. To explore how force can be used to increase yields; decrease solvent use; and alter selectivity, we will study nucleophilic reactions under applied force. We will combine experimental organic chemistry with reactor design and density-functional theory to study how force drives reactivity and selectivity in an effort to develop new rules that anticipate product distributions in these essential reactions.>
Participating Sites: TAMU and UPenn (Experimental); UPenn and UC Merced (Computational)
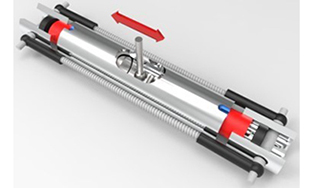
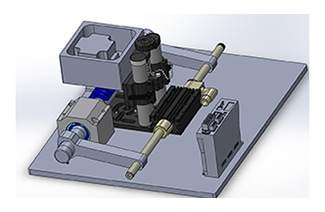
Conceptually, the principle of mechanochemistry is simple: applying forces on reactants to make them look like the desired product will enhance reaction rates, while forces that impede reactants from taking the form of the product retards reaction rates. Although conceptually straightforward, significant challenges remain to experimentally control how the application of macroscale force imparts forces at the atomic scale, and to further predict how these forces drive reactions computationally. This project advances the state-of-the-art of experimental and computational toolsets used to predict and drive mechanochemical reactions, including developing reactors with integrated force control, exploring the application of force in ab initio density functional theory, and atomic scale interfacial reactivity in molecular dynamics. Work will involve the development of new mechanochemical reactors and the introduction of new appraoches for in situ reaction monitoring.
Participating Sites: Univ. of Cincinnati and Vanderbilt (Experimental); UC Merced (Computational)

A popular method to enable or dramatically increase mechanochemical reactions is to add a small amount of solvent to the reaction vessel during grinding. Despite the broad success of liquid assisted grinding (LAG), the fundamental mechanisms that drive the increase in reactivity is still unclear. Students working on this project will investigate specifically how coordinated solvents assist the mechanochemical reaction of metal salts, taking into account milling conditions and different degrees of solvent coordination. The outcome of this work will provide key insights into solvent selection to optimize liquid assisted grinding reactions.
Participating Sites: UPenn, Univ. of Cincinnati, Vanderbilt, William & Mary and CUNY (Experimental); UC Merced and UPenn (Computational)
A key feature of mechanochemistry is that force has both a magnitude and a direction relative to the orientation of molecular bonds in a reaction, as opposed to solvothermal reactions that are directionally isotropic. However, due to toolset limitations, measuring the effect of the applied force direction on large scale chemical synthesis is relatively unexplored. This project will utilize unconventional tools to explore the role of the direction of applied force on reactivity, where both hydrostatic pressure and shear are systematically controlled during reaction. The outcomes of this project will provide key insights into how designers of mechanochemical reactions can utilize force direction to optimize reactions.
Participating Sites: TAMU, Vanderbilt, Univ. of Cincinnati and William & Mary (Experimental)
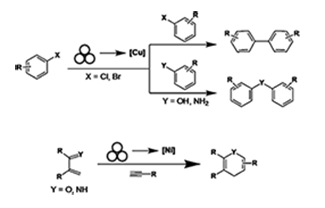
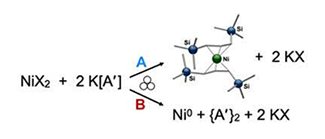
Transition metal catalysis has expanded opportunities in synthetic chemistry by enabling new types of reactivity. Catalysts using metals such as palladium, platinum, iridium, and rhodium have pushed the boundaries of synthetic organic chemistry but are typically costly. The use of abundant metals such as copper, iron, and nickel in solution chemistry has found utility in organic synthesis but at times can require exotic ligands that can be expensive from both a financial and time perspective. The study for this project is to use mechanochemical reaction conditions to drive catalysis at the surface of metals to reduce the cost and the preparation time devoted towards using these metals. These “mechanocatalytic” conditions will be used to prepare various organic and organometallic compounds in a new, sustainable method using bulk metal sources and custom designed equipment.
Participating Sites: TAMU (Computational)
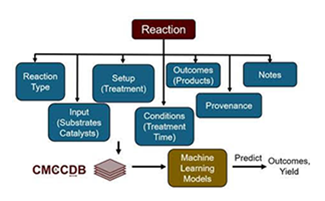
Many synthesis and catalysis processes have recently been accelerated with machine-learning-driven optimization techniques. These optimization techniques have unique challenges in chemistry, due to the combination of both continuous parameters (such as temperature) and discrete parameters (such as catalyst or solvent identity). However, most current approaches only use data to optimize the process and generally are not aware of the fundamental chemical principles of the reaction. In this project, we will work to integrate physically-motivated information about the system (e.g., reaction barrier heights, local measures of chemical structures) to accelerate the optimization of mechanochemical reactions. We will start by building off of the repository of known reactions already conducted by the center and then conduct an optimization for a new set of reactions within this class. If time permits, we will then test the capability of these models to transfer knowledge to successively more diverse substrates.
Participating Sites: TAMU and CUNY (Computational)
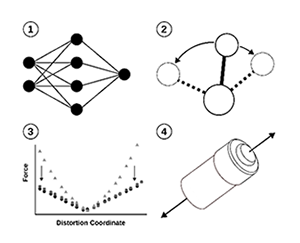
This project applies machine learning techniques to accelerate the discovery of new chemical reactions. Computational methods are essential for understanding which reactions are favorable and how molecular components or reaction conditions can be optimized to improve yields or rates. However, traditional approaches are often too computationally demanding to explore the vast chemical space of potential reactions. To address this, we will develop an active learning framework that combines low-cost machine learning models for initial reaction screening with first-principles calculations for validation. Participants will gain experience in computational chemistry, machine learning workflows, and the fundamentals of mechanochemistry. As this is a rapidly evolving area of development in the center, the specific target reactions may vary but could include cyclization, single-bond formation, substitution, and elimination reactions.
Participating Sites: William & Mary and Univ. of Cincinnati (Experimental)

Mechanochemistry’s furthered adoption towards complex total synthesis remains a challenge for chemists. Natural product molecules have seen their total syntheses tackled by the implementation of emerging technologies such as photochemistry, electrochemistry, and flow chemistry. This study will utilize mechanochemistry to execute the total synthesis of Honokiol in a solvent-free or solvent-minimal pathway. Throughout the route’s development, new reaction methodologies may need to be implemented and explored. This work will be performed in tandem between the Speight and Mack groups which will have full utility to explore various mechanochemical tools such as ball-milling, resonant acoustic mixing, and extrusion.
Participating Sites: William & Mary, TAMU, UPenn (Experimental)
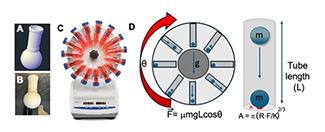
The cost of mechanochemical equipment can often be prohibitive for its use in education and for new research groups. These high costs tend to lead researchers to see mechanochemistry as a “high risk, high reward” area of research. This project seeks to develop low-cost mechanochemical methods for synthesis by transforming affordable or accessible laboratory equipment into mechanochemical reactors. Through the development of 3D printed reaction vessels, we can design new systems that can be equipped to tools such as tube rotators or rotary evaporators. In this project, design efficacy will be evaluated using a series of test reactions (e.g. the Knoevenagel condensation reaction between vanillin and barbituric acid and the Cu(II) catalyzed coupling of isocyanate and sulfonamide), whereby yield optimization will be used to guide reactor vessel design to reveal how the mechanics of the materials printing materials used for the reactors, and the 3D printed reactor size and shape alters performance. This work will be performed in the Speight and Batteas labs with consultation from the Carpick and Felts Labs. Advanced 3D print manufacturing with versatile materials will be accessed via the Carpick and Felts labs.
Participating Sites: TAMU (Experimental) and Northwestern Univ. (Computational)
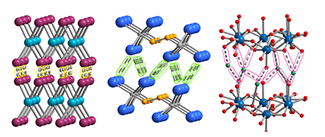
Counterintuitively, applying mechanical force can induce phase transitions in solid-state materials, transforming three-dimensional network structures into two-dimensional layered frameworks with ordered void spaces. These anisotropic materials are promising platforms for developing for next-generation electronic technologies. Our project aims to leverage this chemistry using an integrated approach that combines synthesis, advanced characterization and computation. Specifically, we will evaluate how different mechanochemical methods influence structure formation in lanthanide-containing systems predisposed to anisotropy. Together, these efforts will establish new synthetic routes for tuning electronic behavior, opening pathways to previously inaccessible electronic and magnetic properties.
Participating Sites: TAMU (Experimental)
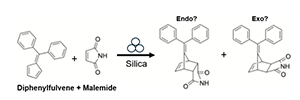
Mechanochemical reactions proceed via the introduction of mechanical forces via milling, shaking or shearing, and the means by which these forces are distributed can influence overall reaction rates, product yields, and selectivity. In this project, students will examine the impact of inert milling additives (such as silica particles) on reactions such as, the Diels-Alder reaction between maleimide and diphenylfulvene, which has been shown in the past to be accelerated in the presence of additives, however the distribution of forces at play and the role of additive particle size on the synthesis has yet to be established. To that end, in this project students will carry out this reaction modifying both milling environment and milling media to determine the impacts of milling forces on reaction outcomes. Students will also learn methods to track the progress of these mechanochemical reactions and characterize reaction products such as nuclear magnetic resonance (NMR) spectroscopy and powder X-ray diffraction (PXRD). Reactions will be compared in both ball milling and resonant acoustic mixing (RAM).
Participating Sites: Northwestern Univ. (Computational)
This project explores how pressure and shear can be leveraged to enable new synthesis routes for solid-state materials. Traditional approaches rely on thermodynamics, but mechanochemical effects—such as stress-induced changes in reaction pathways—are often ignored. The REU student will help develop computational tools that quantify “mechanochemical advantage” by integrating pressure-dependent properties and activation volumes into synthesis prediction algorithms. The REU student will contribute to a scalable framework for predicting mechanochemically selective synthesis routes, advancing discovery of functional materials for energy, catalysis, and quantum technologies.
Participating Sites: Northwestern Univ. (Computational)
Mercury carbodiimide (HgNCN) demonstrates pressure-induced control over mesomeric bonding, enabling transitions between distinct electronic states. This REU project will extend this concept by identifying and investigating other compounds that exhibit similar pressure-tunable bonding and electronic properties. Students will use computational methods (e.g., density functional theory) and literature surveys to predict candidate materials, focusing on systems where mechanochemical stress can induce structural or electronic phase changes. The goal is to discover new materials with tunable properties for applications in electronics and energy storage.