

What are the rules that govern mechanically driven reactions? Can we measure them? Can we control them sufficiently to direct specific reaction outcomes? These are the basic questions at the heart of the research conducted within the CMCC.
The current state-of-the-art in mechanochemical synthesis is not much more sophisticated than mixing reactants together and hitting them with a hammer. While it has become apparent from the last few decades that you can hit a surprising amount of things with a hammer to create seemingly limitless chemical products, prediction is significantly limited. What happens if someone hits the reactants harder or softer, hits them head on or with glancing blows, hits them once or millions of times? And if these parameters matter, can we even make a hammer that can control them?
The CMCC has set about to begin to answer these questions through two research thrusts (RTs): 1) Mechanochemically-Driven Organic Reactions and 2) Oriented Stress for Solid-State Synthesis. Inter-connected with these thrusts is the research and development of a bold suite of experimental and computational tools (the Interdisciplinary Toolset Program) that are essential to unlocking the secrets of mechanically-driven reactivity.
The current state-of-the-art in mechanochemical synthesis is not much more sophisticated than mixing reactants together and hitting them with a hammer.
The experimental methods to be employed and developed will involve hybrid surface analytical tools that will have the capacity to exert precisely-controlled forces, measure the influence of force on reaction kinetics, probe atomic-scale through mesoscale structures, and monitor surface chemical changes and product distributions from force-driven reactions. The suite of experimental tools span the length and time scales needed to study force-driven surface chemical reactions in well-controlled environments encompassing vacuum, gas, and liquid, and will enable the design of scaled-up of next generation mechanochemical reactors with control over reaction rates and product selectivity.
No predictive guidance exists for reactions under shear or compression.
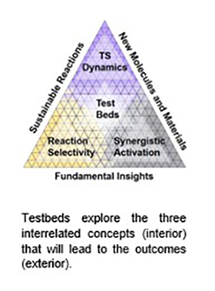
CMCC will explore gaps in the understanding of the three interrelated concepts that dictate reactivity in mechanically-driven organic reactions: (1) transition state (TS) dynamics, (2) reaction selectivity, and (3) synergistic activation–the combination of force with other forms of energy. This will be accomplished through the 8 chemical testbeds which are chemical reactions that are carefully selected to interrogate these three interrelated concepts. Experimental and theoretical tools from the ITP will explore and quantify how force affects reaction kinetics and selectivity. With these quantitative data, we will develop "linear free energy relationships," like Hammett plots, to transform mechanochemistry from its heavily empirical basis to one founded on predictive science. The reactions will be logged into the mechanochemical reaction database, which will be used in the transfer learning training of machine learning models for "forward" mechanochemical reaction prediction.
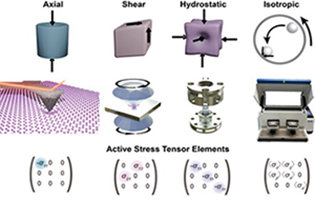
Stress is an untapped actor for creating new chemical bonds. Moving beyond hydrostatic pressure to realize the full potential of stress to drive chemical transformations requires convergence of mechanical engineers, chemists, and materials scientists. Stress σij is a symmetric second-rank tensor comprising at most 6 independent components, each offering a different lever for effecting chemical transformations. We will target bond breaking and formation – the core of chemical synthesis – through four different states of stress. Axial, shear, and hydrostatic pressure states provide unique separation of anisotropic applied forces, whereas ball milling mixes force components, sampling them from a distribution that depends on the milling parameters and thus is represented by time-averaged stress components. These different approaches provide access to elastic and inelastic regimes that enable identification of mechanochemical mechanisms operating at the atomic scale.Stress is an untapped actor. . .