
ch1001.doc
Chapter 10. Reactions in Aqueous Solutions I: Acids, Bases and Salts
|
1. General Properties of Acids and Bases |
|
2. The Arrhenius Theory |
|
3. Hydrated Hydrogen Ion |
|
4. The Bronsted-Lowry Theory |
|
5. Acid Strengths |
|
6. Acid and Base Reactions |
|
7. Acid and Basic Salts |
|
8. Amphoteric substances |
|
9. Preparation of Acids |
|
10.The Lewis Theory |

Svente August Arrhenius
1859 - 1927 (Stockholm)
Ostwald; Boltzman; van't Hoff
Nobel Prize 1903
"Electrical Conductivity
of Solutions"
ch1002.doc
Acid
|
· hydrogen ions when dissolved in water |
|
· strong acids: release lots |
|
· hydrochloric, nitric, sulfuric |
|
· weak acids: release little |
|
· sour taste of lemons, vinegar, and food |
|
· compounds of acidic oxide and water |
|
· liberate carbon dioxide from marble |
|
· extremely useful: |
|
body lotion, candy, coffee, soft drinks, lemonade, mouthwash, orange juice, perfume, pickles, smokeless tobacco, vinegar, DNA, car battery, etching processes, murder, chemical research, titrations, drinks, metal cleaners, brick washing, cologne, deodorants, baby formulas, Aspirin, etc... |
ch1002.doc
|
ACIDS |
|
|
taste |
sour |
|
indicator reaction |
blue litmus ® red |
|
action on metals |
liberate H2 |
|
action on metal oxides |
form salts + H2O |
|
action on salts |
liberate the acid |
|
conductivity |
yes |
|
BASES |
|
|
taste |
bitter |
|
indicator reaction |
red litmus ® blue |
|
action on metals |
liberate H2 from Al |
|
action on protonic acids |
form salts + H2O |
|
action on skin |
slippery |
|
conductivity |
yes |
ch1003.doc page2
Examples:
|
action on metals |
liberate H2 |
2 HCl(aq) + Mg(s) ® H2(g) + MgCl2(aq) magnesium chloride
3 H2SO4(aq) + 2 Al(s) ® 6 H2(g) + Al2(SO4)3(aq) aluminum sulfate
2 NaOH(aq) + 6 H2O + 2 Al(s) ® 3H2(g) + 2 NaAl(OH)4 sodium aluminate
|
action on metal oxides |
form salts + H2O |
2 HCl(aq) + CaO(s) ® CaCl2(aq) + H2O
ch1003.doc page3
|
action on salts of weaker acids |
liberate the acid |
HCl(aq) + CH3COONa(aq) ® CH3COOH(aq) + NaCl acetic acid
|
action on protonic acids |
form salts + H2O |
NaOH(aq) + HCl ® NaCl + H2O
3 Ca(OH)2(aq) + 2 H3PO4 ® Ca3(PO4)2 + 6 H2O
ch1004.doc
Arrhenius definition of:
|
ACID |
|
|
|
contains hydrogen |
|
|
liberates H+, protons, or hydrogen ions |
|
BASE |
|
|
|
contains OH- |
|
|
liberates hydroxide ions in aqueous solution |
|
NEUTRALIZATION |
|
|
|
combination of H+ ions with OH- ions |
|
|
H+ (aq) + OH- (aq)® H2O(l) |
Terms revisited:
|
ELECTROLYTE |
strong and weak |
|
DISSOCIATION |
partial and complete |
|
|
|
|
STRONG ACIDS: |
HCl, HBr, HI, H2SO4, |
|
|
HNO3, HClO4, HClO3 |
|
|
|
|
WEAK ACIDS: |
HF, HCN, H2CO3, |
|
|
H2SO3, H3PO4, CH3COOH |
|
|
|
|
STRONG BASES: |
LiOH, NaOH, KOH, |
|
|
RbOH, CsOH |
|
|
Ca(OH)2, Sr(OH)2, Ba(OH)2 |
Review Sections 4-2; 4-5; 6-7
ch1004.doc page2
EXAMPLES
Neutralization:
HCl(aq) + NaOH(aq) ® H2O(l) + NaCl(aq) (formula unit)
[H+ (aq) + Cl-(aq)] + [Na+(aq) + OH-(aq)] ® [Na+(aq) + Cl-(aq)] + H2O(l) (total ionic)
H+(aq) + OH-(aq) ® H2O(l) (net ionic)
Strong Electrolyte:
HCl(g) + H2O(l) ® H+(aq) + Cl-(aq)
Weak electrolyte:
HF(g) + H2O(l) ¬ ® H+(aq) + F-(aq)
Strong bases:
[Na+ OH-](s) + H2O ® Na+(aq) + OH-(aq)
Weak acid:
CH3COOH(l) + H2O ¬ ® CH3COO-(aq) + H+(aq)
acetic acid acetate proton
99% 1%
ch1005.doc
In aqueous solution the proton or hydrogen ion is hydrated
H+(H2O)n where n ~ 4
HYDRONIUM ION, H3O+

|
hydronium ion |
hydrated hydronium ion |
H+ will always imply a hydrated proton
H+ will always imply a hydrated hydronium ion
ch1006.doc
Bronsted theory or Bronsted-Lowry theory
ACID: H+ proton donor
Any substance which by its own ionization or in a reaction can furnish a proton
|
Acid ® base + H+ |
|
|
|
H3O+ ® H2O + H+ |
|
|
HCl ® Cl- + H+ |
|
|
HSO4- ® SO42- + H+ |
BASE: H+ proton acceptor
|
Base + proton ® acid |
|
|
|
NH3 + H+ ® NH4+ |
|
|
OH- + H+ ® H2O |
|
|
C2H3O2- + H+ ® HC2H3O2 |
·
No great concentration of H+ch1006.doc
An acid-base reaction involves the transfer of a proton from an acid to a base:

Conjugate acid-base pairs: differ by a proton

ch1006.doc page2
BRONSTED THEORY EXAMPLES(proton donor-proton acceptor)
|
HA |
+ |
B |
® |
HB+ |
+ |
A- |
|
acid |
|
base |
|
conjugate acid |
|
conjugatebase |
|
strong acid: |
|
|
|
|
|
|
|
HCl |
+ |
H2O |
® |
H3O+ |
+ |
Cl- |
|
acid |
|
base |
|
conjugate acid |
|
conjugate base |
|
weak acid: |
|
|
|
|
|
|
|
CH3COOH |
+ |
H2O |
® |
CH3COO- |
+ |
H3O+ |
|
acetic acid |
|
base |
|
acetate |
|
conjugate acid |
|
weak base: |
|
|
|
|
|
|
|
HCl |
+ |
:NH3 |
® |
NH4+ |
+ |
Cl- |
|
acid |
|
base |
|
conjugate acid |
|
conjugate base |
|
water as acid: |
|
|
|
|
|
|
|
H2O |
+ |
NH3 |
® |
NH4+ |
+ |
OH-- |
|
acid |
|
base |
|
conjugate acid |
|
conjugatebase |
ch1007.doc
AUTOIONIZATION of AMPHIPROTIC MOLECULE H2O
H2O + H2O = H3O+ + OH--

H2O = H+ + OH- (really a pair of equilibrium arrows
ch1008.doc
Acid Strengths of Hydrohalic Acids in the gas phase:
HF(weakest acid) << HC l< HBr < HI (strongest acid)
Acid Strengths of Hydrohalic Acids aqueous solution of HX:
HF is weak
HCl, HBr, HI (100% ionized) all very strong.
Because of LEVELING EFFECT no differentiation in acid strengths is possible.
H3O+ is the strongest acid in water solution
OH- is the strongest base. In water: NH2- + H2O ® NH3 + OH-

ch1008.doc
(the = sign represents an equilibrium ; one arrow facing right over the other arrow facing left)
Relative Strengths of Conjugate Acid-Base Pairs
HClO4 and HNO3 are strong acids
|
HClO4 |
+ |
H2O |
= |
H3O+ |
+ |
ClO4- |
|
strong acid |
|
weak base |
|
strong acid |
|
weak base |
|
HNO3 |
+ |
H2O |
= |
H3O+ |
+ |
NO3- |
HCN and H2O are weak acids
|
HCN |
+ |
H2O |
= |
H3O+ |
+ |
CN- |
|
weak acid |
|
weak base |
|
strong acid |
|
strong base |
|
H2O |
+ |
H2O |
= |
H3O+ |
+ |
OH- |
NH3 is a very weak acid
|
NH3 |
+ |
H2O |
= |
H3O+ |
+ |
NH2- |
|
weak acid |
|
weak base |
|
strong acid |
|
very strong base |
ch1009.doc
(the = sign represents an equilibrium ; one arrow facing right over the other arrow facing left)
Some typical ionizations:
|
|
HOH |
= |
H+ |
+ |
OH- |
|
|
HCl |
® |
H+` |
+ |
Cl- |
|
|
HClO4 |
® |
H+ |
+ |
ClO4- |
|
|
HNO3 |
® |
H+ |
+ |
NO3- |
|
|
NH4+ |
= |
H+ |
+ |
NH3 |
All of the above involve H2O:
|
|
HX |
+ |
H2O |
= |
H3O+ |
+ |
X- |
|
or more explicitly: |
|
|
|
|
|
|
|
|
|
HX(aq) |
+ |
H2O(l) |
= |
H3O+(aq) |
+ |
X- (aq) |
ch1009.doc
(the = sign represents an equilibrium ; one arrow facing right over the other arrow facing left)
Reactions of ACIDS + BASES. All involve
H3O+ + OH- = 2H2O and usually form salts:
HCl + NaOH = NaCl + H2O
CH3COOH + NaOH = CH3COONa + H2O
Neutralization of Perchloric acid. Its Total Ionic Equation:
[H+(aq) + ClO4- (aq)] + [Na+(aq) + OH- (aq)] ® [Na+(aq) + ClO4- (aq)] + H2O(l)
ch1009.doc
Neutralization of insoluble hydroxide. Example:
formula unit:
Mg(OH)2(s) + 2 HCl(aq)® MgCl2(aq) + 2H2O(l)
total ionic:
Mg(OH)2(s) + 2 [H+(aq) + Cl-(aq)] ® [Mg2+(aq) + 2 Cl-(aq)] 2 H2O(l)
net ionic:
Mg(OH)2(s) + 2 H+(aq) ® Mg2+(aq) + 2 H2O(l)
ch1009b.doc
Focus on stoichiometry of neutralization
Neutralization Reactions formula unit examples
NaOH(aq) + HBr(aq) ® NaBr(aq) + H2O(l)
2NaOH(aq) + H2SO4(aq) ® Na2SO4(aq) + 2 H2O(l)
3NaOH(aq) + H3PO4(aq) ® Na3PO4(aq) + 3 H2O(l)
Ba(OH)2(aq) + 2 HBr(aq) ® BaBr2(aq) + 2 H2O(l)
Ba(OH)2(aq) + H2SO4(aq) ® BaSO4 (s) + 2 H2O(l)
3 Ba(OH)2(aq) + 2 H3PO4(aq) ® Ba3(PO4)2(s) + 6H2O(l)
NH3(aq) + HBr(aq) ® NH4Br(aq)
2 NH3(aq) + H2SO4(aq) ® (NH4)2SO4(aq)
ch1010.doc
Acidic Salts: Not enough base added
formation of: sodium dihydrogen phosphate
H3PO4(aq) + NaOH(aq) ® NaH2PO4(aq) + H2O(l)
formation of: disodium hydrogen phosphate
H3PO4(aq) + 2 NaOH(aq) ® Na2HPO4(aq) + 2H2O(l)
formation of sodium phosphate (normal salt)
H3PO4(aq) + 3 NaOH(aq) ® Na3PO4(aq) +H2O(l)
ch1010.doc
Basic Salts: Not enough Acid added
formation of: Aluminum dihydroxide chloride
Al(OH)3(s) + HCl(aq) ® Al(OH)2Cl(s) + H2O(l)
formation of: Aluminum hydroxide dichloride
Al(OH)3(s)+2HCl(aq)® Al(OH)Cl2(s) +2H2O(l)
formation of: Aluminum chloride (normal salt)
Al(OH)3(s)+3 HCl(aq) ® AlCl3(aq) + 3 H2O(l)
ch1010.doc page2
Partial Neutralization
Sodium Hydroxide and sulfuric acid:
NaOH + H2SO4 ® H2O + NaHSO4 sodium bisulfate (acidic salt)
2 NaOH + H2SO4 ® 2 H2O + Na2SO4 sodium sulfate (normal salt) strong electrolyte

KHP:is a Primary Standard in titrimetry.
ch1010.doc
Normal, Acidic and Basic Salts
|
sulfuric acid |
H2SO4 |
|
potassium sulfate |
K2SO4 |
|
potassium hydrogen sulfate |
KHSO4 |
|
|
|
|
arsenic acid |
H3AsO4 |
|
sodium arsenate |
Na3AsO4 |
|
disodium hydrogen arsenate |
Na2HAsO4 |
|
sodium dihydrogen arsenate |
NaH2AsO4 |
|
|
|
|
hydrochloric acid |
HCl |
|
lithium chloride |
LiCl |
|
Possible ions: |
|
|
|
|
|
|
|
Cations |
Na+ |
H+ |
K+ |
Li+ |
|
|
Anions |
SO42- |
HSO4- |
Cl- |
|
|
|
|
AsO43- |
HAsO42- |
H2AsO4- |
|
ch1010.doc
Normal, Acidic and Basic Salts
|
carbonic acid |
H2CO3 |
|
sodium bicarbonate |
NaHCO3 |
|
(sodium hydrogen carbonate) |
|
|
|
|
|
lead hydroxide |
Pb(OH)2 |
|
basic lead nitrate |
Pb(OH)(NO3) |
|
(lead hydroxide nitrate) |
|
|
|
|
|
bismuth hydroxide |
Bi(OH)3 |
|
basic bismuth nitrate |
Bi(OH)2NO3 |
From Dial Soap Label:
sodium tallowate, sodium cocoate,....
sodium chloride (NaCl), . titanium dioxide (TiO2)
chromium hydroxide green (Cr(OH)3)
ch1011.doc
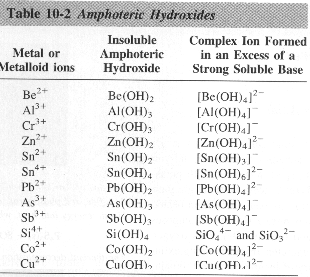
AMPHOTERIC SUBSTANCE: can react as an acid or base
AMPHIPROTIC SUBSTANCE:accept or donate a proton
Al(OH)3(s) + 3 HCl(aq) ® AlCl3 + 3 H2O
Al(OH)3(s) + NaOH(aq) ® NaAl(OH)4(aq)
CH1012.doc
PREPARATION of ACIDS
·
Sulfuric acid + Salt: ® volatile acids: HCl, HBr, HI·
Non-metal oxides + Water (no oxidation change)·
Non-metal halides + Water (hydrolysis)phosphorus pentachloride® phosphoric acid
PCl5 + 4 H2O ® O=P(OH)3 + 5 HCl or written slightly differently
(OH)3P(OH)2 ® (OH)3P=O + H2O
ch1013arsenic(III)bromide arsenious hydroxide
AsBr3 + 3 H2O ® As(OH)3 + 3 HBr (or H3AsO3)
|
Arhenius |
|
|
Bronsted-Lowry |
|
|
Lewis |
|
|
LEWIS ACID: |
eletron pair acceptor |
|
LEWIS BASE: |
electron pair donor |
|
NEUTRALIZATION: |
formation of a coordinate covalent bond; |

ch1014.doc
LEWIS


Oxidative addition accompanied by change in both electronic and molecular geometry

ch1015.doc
Summary:
|
1884 Arrhenius |
|
|
|
|
|
acid: |
proton |
|
|
|
base: |
hydroxide |
|
|
|
|
neutralization: |
H+ + OH- ® H2O |
|
|
|
|
|
|
1923 Brønsted |
|
|
|
|
|
acid: |
proton donor |
|
|
|
base: |
proton acceptor |
|
|
|
|
neutralization: |
H+ + base ® Hbase+ |
|
|
|
(proton transfer) |
|
|
|
|
|
|
|
1923 Lewis |
|
|
|
|
|
acid: |
electron pair acceptor |
|
|
|
base: |
electron pair donor |
|
|
|
|
neutralization: |
electron pair transfer |
|
|
|
(coordinate covalent |
|
1 8268sum.doc
chaptitl.doc
Chapter Titles geouped by ___________
|
1. Foundations of Chemistry |
|
|
|
5. The Structure of Atoms |
|
|
|
10. Reactions in Aqueous Solutions I: Acids, Bases, Salts |
|
|
conjug10.doc
In connection with the theory of conhugate acids and conjugate bases.
con·ju·gate
(k¼n"j…-g³t") v. con·ju·gat·ed, con·ju·gat·ing, con·ju·gates. --tr. 1. Grammar. To inflect (a verb) in its forms for distinctions such as number, person, voice, mood, and tense. 2. To join together. --intr. 1. Biology. To undergo conjugation. 2. Grammar. To be inflected. --con·ju·gate (-g¹t, -g³t") adj. 1. Joined together, especially in a pair or pairs; coupled. 2. Mathematics & Physics. Inversely or oppositely related with respect to one of a group of otherwise identical properties, especially designating either or both of a pair of complex numbers differing only in the sign of the imaginary term. 3. Chemistry. Pertaining to an acid and a base that are related by the difference of a proton. --con·ju·gate (-g¹t, -g³t") n. Mathematics & Physics. Either of a pair of conjugate quantities. --con"ju·gate"ly adv. --con"ju·ga"tive adj. --con"ju·ga"tor n.
end of chapter 10 notes