Pages 134-137 of your textbook include the rules you'll need when writing formula unit, total ionic, and net ionic equations. The following summarizes this information. Please look in your book for the details.
Formula Unit Equations show complete formulas for all compounds. An example is the reaction of aluminum and potassium hydroxide in Experiment 6: Recycling Aluminum Cans.
2Al (s) + 6H2O (l) + 2KOH (aq) ® 2K[Al(OH)4] (aq) + 3H2 (g)
Total Ionic Equations give formulas written to show the predominant form each substance exists as when in contact with water (aqueous). So, you break up only the strong electrolytes. (The weak electrolytes like HCN are soluble, but they don't break up into ions very much. In the above reaction, the KOH is a soluble metal hydroxide, so it is a strong electrolyte and it breaks up into its ions. It will be rewritten as K+ + OH-. The K[Al(OH)4] has one potassium ion ionically bonded to a Al(OH)4- ion. It is a soluble salt, but with a twist. In solution, the salt dissolves and forms K+ + Al(OH)4-. Please note that it is NOT rewritten as K+ + Al3+ + (OH)44- or 4OH-. This is because the Al(OH)4- does not dissociate in the aqueous solution but stays together as a complex ion with a -1 charge. The examples and rules for what does or does not dissociate/ionize are given in tables 4-8 and 4-9 on page 135 in your textbook. The book shows brackets, but you don't need them. The brackets may be included to indicate which species would otherwise form a compound when not in water, but I think it is confusing, since in the solution, the ions don't have anything to do with each other. The results are therefore:
2Al (s) + 6H2O (l) + 2K+ (aq) + 2 OH- (aq)] ® 2K+ (aq) + 2Al(OH)4- (aq) + 3H2 (g)
A Net Ionic Equation will show only the species that are involved in the reaction. You'll need to cancel out any spectator ions - species that are absolutely identical in formula and charge from both sides of the equation. These ions are present in the solution, they just aren't involved in the reaction. In the above reaction, compare the left side of the equation to the right side. The first species on the left is Al (s). Looking at the right side of the equation you can see that the Al (s) is now 2Al(OH)4- (aq). It has therefore reacted and must be included. The same with the 6H2O (l). The 2K+ (aq), however, appears unchanged on both sides of the equation. It did not react and can be left out of the equation - it's a spectator.
2Al (s) + 6H2O (l) + 2OH- (aq) ® 2Al(OH)4- (aq) + 3H2 (g)
I would highly recommend that when you are asked to write a net ionic equation, you go ahead and write the formula unit equation first, then the total ionic equation, and finally the net ionic equation. This will save you from making mistakes and help reinforce these ideas.
Another example would be to react HCl with Ba(OH)2.
2HCl (aq) + Ba(OH)2 (aq) ® BaCl2 (aq) + 2H2O (l) formula unit
2H+ (aq) + 2Cl- (aq) + Ba2+ + 2OH- (aq) ® Ba2+ (aq) + 2Cl- (aq) + 2H2O (l) total ionic
2H+ (aq) + 2OH- (aq) ® 2H2O (l) net ionic
Dividing through by 2, we have
H+ (aq) + OH- (aq) ®
H2O (l) net
ionic
Note that in this example, the only species that actually react are the hydrogen and hydroxide ions. The other ions in the total ionic equations are considered to be spectator ions. In this example, Ba(OH)2, which is a dibasic base, is used. A dibasic base has two hydroxide ions so that one molecule can accept two hydrogen ions (or protons). A tribasic molecule, for example, would be able to react with 3 hydrogen ions. Consequently, a diprotic acid would be an acid with two hydrogen ions to give such as H2SO4. Examples of other diprotic acids and bases may be found in table 4-9 on page 135.
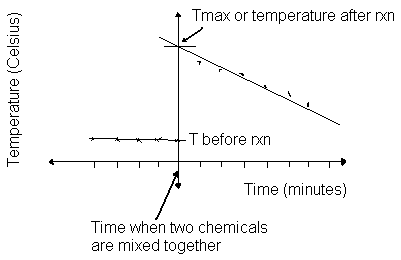
Calorimetry is a technique of determining the amount of heat absorbed (endothermic) or released (exothermic) in a reaction. In lab you will be using a styrofoam cup filled with one reactant - called a coffee cup calorimeter, which works at constant pressure, so the heat measured is related to the enthalpy of the reaction. You will measure the temperature of the reactant, then add another reactant. The two substances will react and there will be a temperature change. You will measure this change for at least 10 minutes to make a graph similar to this one.

Note that in this graph, the first five readings indicate an average temperature before the reaction took place. These temperatures were taken before mixing the two chemicals. After mixing and after the reaction has started, the temperature jumps up and then slowly cools down. Since the temperature went up during the reaction, heat was released, the reaction is exothermic and the enthalpy is a negative number. To determine the maximum temperature in the reaction, you must draw a straight line through your data points to the point where the reaction takes place (do this by hand). This process is called extrapolation. The point where the line intersects the time the two chemicals began reacting (when the solutions were mixed) determines the maximum temperature.
The amount of heat released by such a reaction will spread beyond the water inside the styrofoam cup to the cup and the air around it heating each. For this lab we are going to assume that the amount that is absorbed by the styrofoam cup (the calorimeter) and the outside universe is negligible. Is this really a valid assumption? If we were not making this assumption then we would have to first determine the calorimeter's constant by measuring the amount of heat lost in a known reaction and using the following formula:
| (amount of heat released by reaction) | = | (amount of heat gained by calorimeter) | + | (amount of heat gained by solution) |
After obtaining the maximum temperature of the reaction from your graph you will then be able to determine the DH of the reaction with the following equation:
DHrxn = -[msCs(Tarxn-Tbrxn)]
where ms is the mass of solution in the calorimeter (density of solution is = 1.02 g/mL),
Cs is the specific heat of the solution (3.97 J/g-degree C),
Tbrxn is the temperature of the solution before the reaction takes place,
and Tarxn is the maximum temperature of the solution after the reaction takes place.
Now that you have your DHrxn how will you compare your result with others in the class? If for example one group reacts two moles of HCl with two moles of NaOH will they get the same result as another group that reacts one mole of HCl with one mole of NaOH?
Sample Calculations
1. Given the following reaction calculate the number of moles of magnesium hydroxide that will react completely with 45.0 mL 0.020 M HCl.
Mg(OH)2 + 2HCl ® MgCl2 + H2O
answer below
2. Calculate the enthalpy of reaction if the mass of the water inside the calorimeter was 92.01 g, the temperature of this water before the reaction was 24.9 oC and the temperatures after the reaction were: 46.3 oC, 52.1 oC, 51.6 oC, 51.2 oC, 50.5 oC, and 50.0 oC. Assume the specific heat of the solution is 3.97 J/g-degree C.
answer below
3. If DHrxn for the reaction in 2. was supposed to be - 11.2 kJ, what happened to the extra energy?
answer below
Answers
1. First calculate the number of moles of HCl that we have to start with.
![]()
Now calculate the number of moles of Mg(OH)2 that are needed to react completely with this amount, which is the answer.
![]()
2. Graph the last 5 temperatures and extrapolate back to time of mixing to determine the temperature after the reaction. I found mine to be 52.4 oC. Now use the equation above to get the enthalpy of the reaction.
![]()
DHrxn = -10045 J = -10.0 kJ (to the correct number of significant digits)
3. The extra energy was absorbed by the calorimeter and the outside world.