
Investigation #16:
 | Investigation #16:
|
Definitions
Heat Capacity - amount of energy necessary to raise the temperature of a gram of substance one degree celsius.
Specific Heat of Crystallization or Solidification - amount of energy lost to bring about that change of state in one unit mass
Calorimeter Constant - amount of energy needed to raise the temperature
of the calorimeter one degree celsius.
Concepts
To use a calorimeter, you must first determine the calorimeter's constant. To do this, you must run an experiment with a known specific heat. In this experiment, you used the loss of heat by boiling water. Some of the heat lost by the boiling water is transferred to the room temperature water already in the calorimeter resulting in an increase in the temperature of the water inside the calorimeter. Not all of the heat is transferred to the water however. Some of it is transferred to the calorimeter itself. By comparing the amount of heat gained by the water inside the calorimeter with the amount of heat that the water inside the calorimeter should have gained, the amount of heat gained by the calorimeter can be found.
heat lost by boiling water - heat gained by room temp. water in calorimeter = heat gained by calorimeter
With this, you can then find the calorimeter constant. Again, the only reason you can do this is because we already know the specific heat of water.
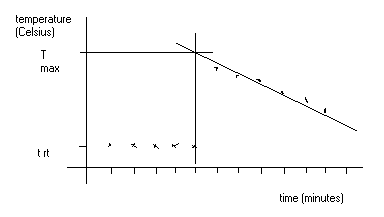
mbCw(Tmax-Tb)
Heat Gained by Room Temperature Water
mrtCw(Tmax-Trt)
Heat Gained by Surroundings (Calorimeter)
mbCw(Tmax-Tb) - mrtCw(Tmax-Trt)
Calorimeter Constant
K = [mbCw(Tmax-Tb) - mrtCw(Tmax-Trt)]/(Tmax-Trt)
Trt = temperature of room temp. water
mb = mass of boiling water
Cw = specific heat of water
mrt = mass of room temperature water
Tb = temperature of boiling water
Tmax = obtained from graph
Now that you have determined the calorimeter constant, you can use the
calorimeter to find for example, the specific heat of solidification for
sodium thiosulfate pentahydrate. A molten solution of sodium thiosulfate
pentahydrate is added to the calorimeter. This molten solution is seeded
with a small crystal which is small enough not to affect the total mass
of the salt greatly. The times and temperatures are again recorded. A graph
is drawn and Tmax determined.
Calculation for D Hsol
D Hsol = -[mwCw(Tmax-Tcw) + K(Tmax-Tcw)
Calculation for D Hsolspec
D Hsolspec = D Hsol/ msalt
mw = mass of water in the calorimeter
Cw = specific heat of water
Tmax = obtained from graph
Tcw = temperature of water in the calorimeter before the vial of salt was added
K = calorimeter constant
msalt = mass of salt
Additional Concepts
When comparing specific heats of solidification, the amount of energy per gram is important but also the melting point range. It is useful to compare materials by energy per gram because it reflects energy/volume. In other words it is also important to know how much energy can be stored in how much space.
You can equate energy lost with energy gained but only if you realize and take into consideration the fact that the values have opposite signs. Energy lost is a negative = Energy gained which is a positive. Here the signs don't work out so you have to add a negative to one side or take the absolute values of both.