2MgAT+ HCO3- + Glutamin+ H2
2MgAD+ P+ Glutamat+ Carbamoyl-
|
CarbamoyPhosphatSynthetas |
CPS catalyzes onothmost complicatereactions iliving systems. This enzymsynthesizes carbamoyl-frobicarbonate, glutamine, antwmolecules oATia reactiothat is illustratebelow.
|
2MgAT+ HCO3- + Glutamin+ H2 |
|
|
|
2MgAD+ P+ Glutamat+ Carbamoyl- |
Thcarbamoyphosphatproduct is subsequently consumeduring thbiosynthesis opyrimidinnucleotides anarginine. Moreover, UMP, thenproduct othpyrimidinbiosynthetic pathway, inhibits thcatalytic reactiowhilornithine, thfirst substratithargininbiosynthetic pathway, allosterically activates thenzyme.
It has beedemonstratethat threactioproceeds via a series ofouseparatreactions with threreactivintermediates, ammonia, carboxy-ancarbamate. Thprogressiooseriaanparallereactions is illustratebelow. essiooseriaanparallereactions is illustratebelow.
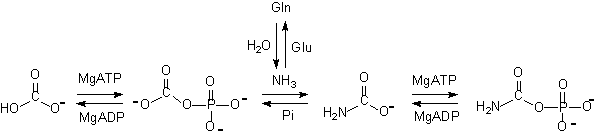
|
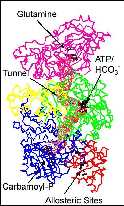
ThenzymfroE. colis founas a heterodimer. ThcarA genencodes thsmalleotwsubunits (Mw = 42,000) whilthcargenencodes a subunit oMw 118,000. Thsmalsubunit is homologous ta diversset oproteins that havbeefountcatalyzthhydrolysis oglutamintglutamatanammonia whilthlargsubunit is a membeothATP-grassuperfamily oenzymes. ThthredimensionacrystastructuroCPS has beesolvethigh resolutioby thlaboratories oHazeHoldeanoIvaRayment at thUniversity oWisconsin. Quitremarkably, ththredimensionacrystastructuroCPS revealethidentity anlocatioothreactivsites connecteby a moleculatunnethat was nearly 100Å ilength! Warexploiting this highly compleproteitanswethfollowing questions.
Thfirst figurillustrates thcomplemoleculaarchitecturoCPS. Thsmalsubunit is coloremagenta whilthfoudomains othlargsubunit arblue, yellow, green, anred. Thmeandering othmoleculatunneconnecting ththreactivsites is presenteas a series orelines.
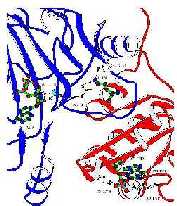
Thseconfigurshows thallosteric domaiirelationshitthcabamoyphosphatdomain. Thbinding sites foIMP/UMP, ornithinanMfATarhighlighted.
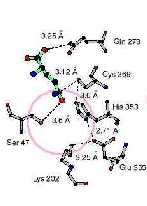
Ththirfigurillustrates thstructurachanges that occuwithithsmalsubunit during thhydrolysis oglutamine. Ithfirst paneis thMichaelis complewith bounglutaminusing thC269S mutant. Thseconpaneshows thtetrahedraintermediatusing thg-glutamysemialdehydas a tightly bouninhibitor. Ththirpaneshows a representatiooththioesteintermediatusing thH353mutant. Alstructures werdonicollaboratiowith ProfessoHazeHolden, University oWisconsin.
 |
 |
 |
Figur1 |
Figur2 |
Figur3 |