Each element on the periodic table consists of protons, neutrons, and electrons. Protons and electrons are charged particles, but neutrons have no charge. The charge of a proton and electron are equal in magnitude, but have opposite signs. We say that protons are positively charged and electrons are negatively charged. Together, protons, neutrons, and electrons make up the mass of the element, which is also called an atom. These subatomic particles are very small, and cannot be seen with the human eye. However, their masses have been measured.
Amu stands for atomic mass unit. Obviously, the mass of a proton and neutron are very similar, while the mass of an electron is much less than the other two particles.
If you know the number of protons, neutrons, and electrons in an element, it is a simple matter to calculate the mass of that element. This brings us to two common terms used in chemistry, atomic number and mass number. The atomic number is the number of protons in an element. The atomic number is what is used to identify the element. The mass number is the number of protons plus the number of neutrons. The elements on the periodic table are organized according to their atomic numbers. See below.
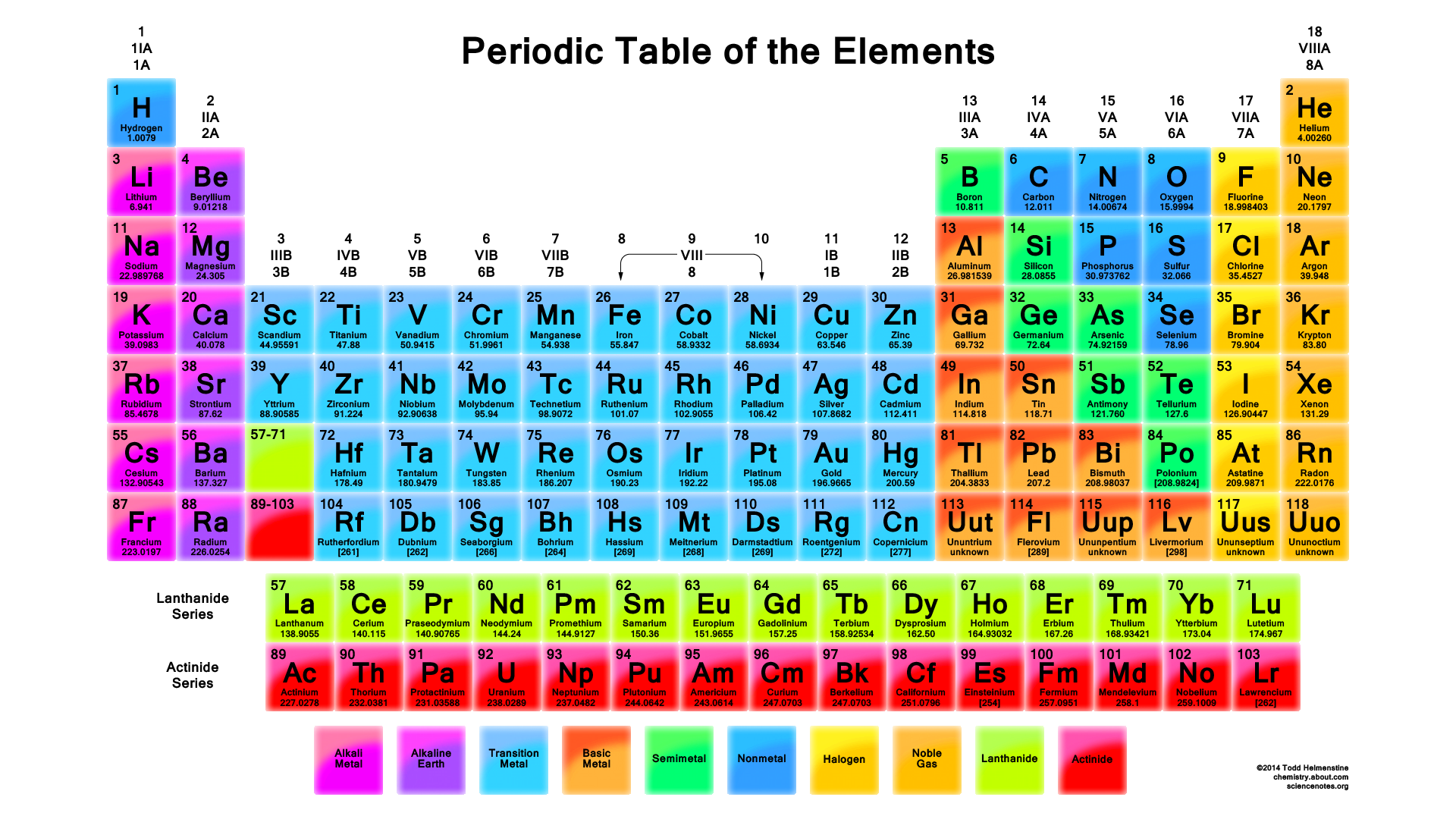
The lightest element on the periodic table is hydrogen; it only contains one proton. Hydrogen will be focused on for much of this introduction.
A hydrogen atom contains one proton and one electron. Therefore, the mass of this element is
There are other numbers that are important on the periodic table, one of which is the atomic mass of the element. This mass is also measured in amu. The atomic mass listed for hydrogen on the period table is 1.00794 amu.
However, there seems to be a discrepancy. The calculated mass of a hydrogen atom is different than the mass reported on the period table. The reason for this discrepancy is that there is another form of hydrogen that exists, one that does not only contain one proton and one electron. This form contains a neutron, so the mass is as follows.
These two forms of hydrogen are called isotopes. Isotopes of an element have the same atomic number, but a different mass number. Hydrogen is the only element that has different names for its isotopes. Hydrogen with one neutron is also called deuterium, and the symbol is D. Hydrogen with two neutrons is called tritium, and the symbol is T.
For each element, each isotope exists in nature in a particular proportion. The percent of a particular isotope that is present is called the percent abundance. Therefore, if a sample of an element is collected, there will be a percentage of each isotope in the sample. These percent abundances have been studied and documented. Here are the percent abundances of the isotopes of hydrogen.
Tritium does not exist in nature, but it can be artificially synthesized.
Let’s get back to the atomic mass on the periodic table. This number takes isotopes into account. The atomic mass is the weighted average of the isotopes of the element. The masses of the isotopes and the percent abundances are used to calculate the atomic mass that is reported on the periodic table. The following is the calculation of the atomic pass of hydrogen.
This mass is comparable to the atomic mass on the periodic table. Obviously, the mass of the heavier isotope contributes more to the atomic mass than the lighter isotope. This calculation can be done for every element; one just needs to know the masses of the isotopes and the percent abundances.
Sample ProblemCalculate the weight averaged atomic mass of hypothetical element X. There are three iosotopes, each with masses 65, 67, and 69. The percent abundances of each isotope, respectively, are 25.654%, 39.156%, and 35.190%.
Answer65(0.25654) + 67(0.39156) + 69(0.35190) = 67.1907
There is a way to write an element symbol that indicates the number of protons and neutrons. A superscript is used for the number of protons and neutrons (the mass number), and a subscript is used for the number of protons (the atomic number). Here are the symbols of the three isotopes of hydrogen.
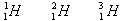
is deuterium and is tritium. Since the superscript is the number of protons and neutrons added together, it is convenient to have a term that describes both types of subatomic particles. A nucleon is a particle that resides in the nucleus, either a proton or neutron. Therefore, the superscript can be said to represent the number of nucleons in the atom.
Determining the number of neutrons in an atom is a simple matter. Consider the following relationship.
number of protons + number of neutrons = mass number
If you are given the symbol of an isotope, then you can rearrange the above equation to solve for the number of neutrons.
mass number – number of protons = number of neutrons
Using this equation, we see that 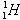 has zero neutrons,
has zero neutrons, 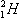 has one neutron, and
has one neutron, and 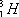 has two neutrons.
has two neutrons.
Finally, it is also appropriate to omit the atomic number in this symbolism. Since the atomic number is the same for any form of an isotope, the subscript does not need to be included. Therefore, the isotopes of hydrogen can be written as:
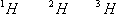
Given: the 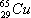 isotope . Calculate the number of neutrons in this isotope of copper.
isotope . Calculate the number of neutrons in this isotope of copper.
Solve: 65 – 29 = 36 neutrons
Answer65(0.25654) + 67(0.39156) + 69(0.35190) = 67.1907