Chapter 13 Notes
IMPORTANT NOTE:
It was impossible to reproduce the large numbers of illustrations found in Chapter 13. (I can't do it in color either) Please refer to the book to review the various book diagrams shown in class.
ch1301.doc
Chapter 13 Liquids and Solids
|
1. Molecular Kinetic Description |
|
2. Intermolecular Attractions |
|
Liquid State |
|
3. Viscosity |
|
4. Surface Tension |
|
5. Capillary Action |
|
6. Evaporation |
|
7. Vapor pressure |
|
8. Boiling Points (Distillation) |
|
9. Heat Transfer |
|
Solid State |
|
10. Melting point |
|
11. Heat transfer |
|
12. Vapor pressure (sublimation) |
|
13. Phase Diagrams |
|
14. Amorphous and Crystalline |
|
15. Crystal structures |
|
16. Bonding |
|
17. Band Theory |
Final exam May 12, 1999 Wednesday 1 pm (Please check accuracy)
14 Chapers to review (542 pages total))
|
If you start reviewing April 21, 1999 |
TheReview Rate: 1.4 days /chapter |
|
If you start reviewing April 28, 1999 |
TheReview Rate: 1.0 Chapters/day |
|
If you start reviewing May 5, 1999 |
TheReview Rate: 2.0 Chapters/day |
|
If you start reviewing May 6, 1999 |
TheReview Rate: 2.3 Chapters/day |
|
If you start reviewing May 7, 1999 |
TheReview Rate: 2.8 Chapters/day |
|
If you start reviewing May 8, 1999 |
TheReview Rate: 3.5 Chapters/day |
|
If you start reviewing May 9, 1999 |
TheReview Rate: 4.6 Chapters/day |
|
If you start reviewing May 10, 1999 |
TheReview Rate: 7.0 Chapters/day |
|
If you start reviewing May 11, 1999 |
TheReview Rate: 14.0 Chapters/day |
ch1302.doc
GASES, LIQUIDS, SOLIDS--Comparison
Gases
1. No definite shape (fill containers completely)
2. Are compressible
3. Have low density
4. Are fluid
5. Diffuse rapidly
6. Consist of extremely disordered particles and much empty space;
particles have rapid, random motion in three dimensions

Liquids
1. Have no definite shape
2. Have definite volume (are very slightly compressible)
3. Have high density
4. Are fluid
5. Diffuse through otherliquids
6. Consist of disordered clusters of particles that are quite close together;
particles have random motions in three dimensions
Solids
1. Have definite shape (resist deformation)
2. Are nearly incompressible
3. Usually have higher density than liquids
4. Are not fluid
5. Diffuse only very slowly threough solids
6. Have an ordered arrangement of particles that are very close together;
particles have vibrational motions only.
ch1303.doc Examples of varying attractive forces:
intermolecular attractions
|
H H |
|
|
H H |
|
|
H H |
|
|
O O |
|
ch1303.doc
INTERMOLECULAR FORCES
· hydrogen bonding
· ion - ion interactions
· dipole dipole interactions
· London Forces
ch1303a.doc
HEAT CALCULATIONS
· heating matter: non-isothermal
· phase change of matter: isothermal
INTERMOLECULAR FORCES
· hydrogen bonding: strongest
· ion - ion interactions: ionic solids
· dipole dipole interactions: liquids
· London Forces: induced polarization
LIQUID STATE
· viscosity
· surface tension
· capillary action
· evaporation
· vapor pressure
· boiling points
SOLID STATE discussed next period
ch1304.doc
INTERMOLECULAR FORCES
HYDROGEN BONDING (O, N. F)
H2O(l) ® H2O(g) requires 40.7 kJ/mole
main attractive force: hydrogen bonding
important: water, alcohols, DNA, amino acids,
H atom is on O, N, F
O¾ H is very polar
bonds with another O, N, or, F
O¾ H¼ ¼ O
hydrogen bond
intermolecular and intramolecular
ch1304.doc
INTERMOLECULAR FORCES
ION-ION attractions:
|
F |
= |
k q1q2 |
|
|
|
|
d2 |
|
|
|
|
|
q1 is charge on the cation |
|
|
|
|
q2 is charge on the anion |
|
|
|
|
d is distance between the charged ion centers |
Ionic compounds with their melting pointsch1304.doc
Trend: Higher molecular weight, more polarization, the charge is more diffuse
hence easier to melt (overcome rigid attractive forces)
|
Salt |
mp |
Salt |
mp |
Salt |
mp |
|
NaF |
993 |
CaF |
1423 |
MgO |
2800 |
|
NaCl |
801 |
Na2S |
1180 |
CaO |
2580 |
|
NaBr |
770 |
K2S |
840 |
BaO |
1923 |
INTERMOLECULAR FORCES
DIPOLE-DIPOLE interactions
Polar Covalent molecules
H2O, NH3, HCl, Ethanol
Molecules with Permanent dipoles
|
CH3 C CH3 |
CHCl3 |
CH2¾ CH2 |
|
acetone |
chloroform |
tetrahydrofuran |
ch1304.doc
INTERMOLECULAR FORCES
LONDON FORCES weak
· freezing and solidifying of inert gases and non-polar atoms
· dipole induced dipole
· 1/d7 dependence (force)
· polarized by nuclei
U µ a 2/d6 (bond energy) polarizability and distance
ch1305.doc
Contributions to overall energy
|
Molecule |
Ar |
CO |
HCl |
NH3 |
H2O |
|
Dipole |
0 |
0.1 |
1.03 |
1.47 |
1.85 |
|
Dipole-dipole energy |
0 |
0 |
3.3 |
13 |
36 |
|
London Energy |
8.5 |
8.7 |
17.8 |
16.3 |
10.9 |
|
Total Energy |
8.5 |
8.7 |
21 |
29 |
47 |
|
Heat of vaporization |
6.7 |
8.0 |
16.2 |
27.4 |
40.7 |
look up: Erudite (µr"y…-dºt", µr"…-) adj.
1. Educated or well-informed; schooled:
|
• knowledgeable |
• scholarly |
|
• literate |
• taught |
|
• educated |
• well-educated |
|
• well-read |
• well-taught |
|
• well-tutored |
• cultivated |
|
• cultured |
• learned |
|
• lettered |
|
ch1305.doc
LIQUID STATE
VISCOSITY - resistance to flow
|
honey - glucose (solution) |
C6H14O6 |
9.1 x 1015 centipoise |
|
glycerine |
C3H8O3 |
1498 centipoise |
|
dodecane |
C12H26 |
1.35 centipoise |
|
pentane |
C5H12 |
0.24 centipoise |
|
water |
H2O |
0.89 centipoise |
|
Iron (1400 oC) |
Fe |
2.25 centipoise |
|
note: Viscosity decreases with a rise in T |
Definition: poise (po¹z) n. A centimeter-gram-second unit of dynamic viscosity
equal to one dyne-second per square centimeter. Unit of Viscosity.
ch13006.doc
SURFACE TENSION:
draws the liquid together and forms the liquid-air interface
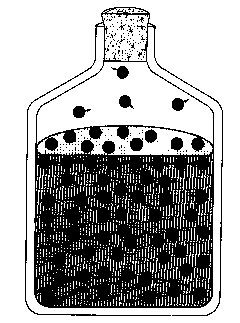
(Surface tension is) a Cohesive force that
Minimizes surface area
explains spherical droplets
bugs walk on water
ch13006.doc
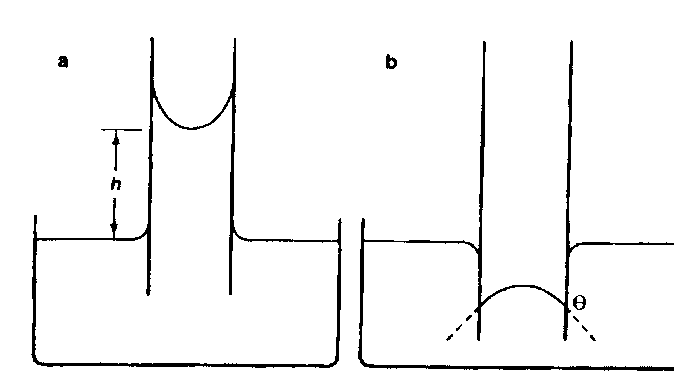
CAPILLARY ACTION:
Adhesive force that causes liquids to rise against gravity up glass tubes
Roots
Meniscus: a) water; b) mercury (in figure)
ch1307.doc Heat
n. Physics. A form of energy associated with the motion of atoms or molecules
and capable of being transmitted through solid and fluid media
by conduction, through fluid media by convection, and through empty space by radiation
Energy (µn"…r-j¶) n. Physics. The capacity of a physical system to do work.
Calorie: heat required to raise 1.00 g H2O 1.00 oC
Joule= 1/0.239 cal. ( 1 Newton meter)
Also 4.184 J =1.000 cal
Specific heat: Heat energy required to raise the temperature of 1.000 g of substance 1.000 oC
|
ice |
2.09 J/g oC (near zero) |
|
water |
4.18 J/g oC |
|
steam |
2.03 J/g oC (near 100) |
ch1307.doc
Q. How much heat is required to warm a cup of coffee at room temperature to 90 oC ?
A. Assume room temperature is 25 oC
Assume a cup holds 250 g of coffee.
Assume coffee is water.
Look up the specific heat of H2O: 4.18 J/g
|
Energy required to heat = D t x mass x specific heat |
Energy = (90-25) x 250 g x 4.18 J/g oC = 67825 J
= 6.78 x 10+4 J
Q. How much heat must be removed to cool a cup of frozen coffee to -10 oC?
A. Look up specific heat of ice: 2.09 J/g oC
Other assumptions as just above.
Heat = (-10 - 0) x 250 g x 2.09 J/g oC = - 5225 J
(negative means heat evolved by system)
= -5.22 x 103 J
ch1307.doc
Molar Heat Capacity
The heat required to raise the temperature of exactly 1 mole of a substance 1.000 oC.
Q. How much heat is required to warm 3.00 mol water at room temperature to 90 oC?
A. 1st, look up or calculate molar heat capacity; 2nd, do the problem. We will actually calculate the molar heat capacity from specific heat
molar heat capacity= 4.18 J/g oC x 18.0 g/mol = 75.2 J/mol oC
Now we solve
Heat = 3.00 mol x 75.2 J/mol oC x 65 oC = 14664 J
Heat of Vaporization: Heat added to a liquid to vaporize 1.000 g at its boiling point.
|
|
boiling point |
specific heat ofvaporization |
|
water |
100 oC |
2260 J/g |
|
ethyl alcohol |
78 oC |
858 J/g |
|
benzene |
80 oC |
395 J/g |
|
carbon tetrachloride |
77 oC |
213 J/g |
Molar Heat of Vaporization: Heat added to a liquid to vaporize 1.000 mol
of liquid at its boiling point: D Hvap .
Q. How much heat is required to vaporize 250 g of water at its boiling point?
A. The process isisothermal at 100 oC: H2O(l) ® H2O(g)
? J = (250 g ) (2260 J/g) = 565 kJ
ch1307.doc
Heat of fusion
Heat of fusion: The heat required to melt 1.000 g of substance at its melting point.
Q. Calculate the heat that must be absorbed in melting 50.0 g ice at its normal melting point?
A. Look up the specific heat of fusion of ice: 334 J/g
50 g x 334 J/g = 16700 J or 16.7 kJ
Molar heat of fusion: The heat required to melt 1.000 mol solid at it's melting point.
?J/mol = 334 J/g x 18 g/mol = 6012 or 6.01 kJ
ch1308.doc
Combined example
A: How much heat is required to convert 10.0 g H2O at 20 oC to water vapor at 120 oC.
Divide into 4 steps and add them up
step 1: Heat the water to 100 oC
10.0 g x (100 - 20 oC) x 4.18 J/g.oC = 3.34 kJ
step 2: vaporize the water at 100 oC
10.0 g x 2.260 kJ/g = 22.6 kJ
step 3: heat the steam to 120 oC
10.0 g x (120 - 100 oC) x 2.03 J/g oC = 0. 407 kJ
step 4: combine individual heats
Total Energy= 3.34 kJ + 22.6 kJ + .407 kJ = 26.4 kJ heat
Q. Finger into the spout of boiling teakettle. Assume that the finger
condenses 0.5 g. steam. How much heat is absorbed by the finger?
The condensation of 1/2 g of water at its boiling point.
? J = 0.5 g x 2260 J/g = 11Z30 J or 1.1 kJ.
ch1309.doc
Summary of Our Understanding of Intermolecular Attractive Forces
Fill in the blanks with HIGH or LOW:
|
Property |
HO-CH2CH2-OH (ethylene glycol) |
CH3-CH3 (Ethane) |
|
Cohesive Forces |
HIGH |
LOW |
|
Viscosity |
HIGH |
LOW |
|
Surface Tension |
HIGH |
LOW |
|
Specific Heat |
HIGHER |
LOW |
|
Vapor Pressure |
LOW |
LOW |
|
Rate of Evaporation |
LOW |
HIGH |
|
Boiling Point |
HIGH |
LOW |
|
Heat of Vaporization |
HIGHER |
LOW |
Practice a table like this for
a) water; b) motor oil; c) gasoline; d) ether; e) nail polish remover; f) turpentine; g) alcohol.
ch1310.doc
SOLIDS Important vocabulary
Solid
Liquid
gas
melting point
normal melting point
freezing
melting
boiling
condensing
endothermic process
exothermic process
temperature
phase (solid, liquid, gas)
Joule
gram
specific heat
heat of fusion
heat of solidification
heat of vaporization
|
6.02 kJ/mol |
+ |
H2O(s) |
® |
H2O(l) (at 0 oC) |
|
|
|
ice |
|
water |
ch1311.doc (water)
Q.How much heat is required to convert
25.0 g of ice at -10.0 oC to steam at 110.0 oC?
step 0: Phase change points and specific heats
Information: m. p. is 0.0 0C, 334 J/g ; b. p. is 100.0 oC 2260 J/g.
Information: specific heats ice 2.09 J/goC; water 4.18 J/g oC; steam 2.03 J/g oC
step 1: Heat ice from -10.0 oC to 0.0 oC
25.0 g x (0.0 --10.0 oC) x 2.09 J/g oC = 522 J
step 2: Melt the ice at 0.0 oC
25.0 g x 334 J/g = 8350 J
step 3: Heat the water from 0.0 oC to 100.0 oC
25.0 g x (100.0 - 0.0 oC) x 4.18 J/g oC = 10450 J
step 4: Vaporize the water to steam at 100.0 oC
25.0 g x 2260 J/g = 56500 J
step 5: Heat the steam from 100.0 oC to 110.0 oC
25.0 g x (110.0 -100.0 oC) x 2.03 J/g oC = 507 J
step 6: combine individual steps
Total heat absorbed = 522 + 8350 + 10450 + 56500 + 507 = 76329 J or 76.3 kJ
ch1312.doc (benzene)
Q. How much heat is required to convert
25.0 g of frozen benzene at -10.0 oC to vapor at 110.0 oC?
step 0: Phase change points and specific heats
m. p. is 5.5 0C, 127 J/g ; b. p. is 80.1 oC 395 J/g.
specific heats frozen benzene 1.10 J/goC; liquid 1.74 J/g oC; vapor 1.04 J/g oC
step 1:Heat solid benzene from -10.0 oC to +5.5 oC
25.0 g x (5.5 --10.0 oC) x 1.10 J/g oC = 426 J
step 2: Melt the solid benzene at 5.5 oC
25.0 g x 127 J/g = 3174 J
step 3:Heat the liq benzene from 5.5 oC to 80.1 oC
25.0 g x (80.1 - 5.5 oC) x 1.74 J/g oC = 3245 J step 4:Vaporize liquid benzene to vapor at 80.1 oC
25.0 g x 395 J/g = 9875 J
step 5:Heat the benz. vapor from 80.1 to 110.0 oC
25.0 g x (110.0 -80.1 oC) x 1.04 J/g oC = 777 J
step 6: compbine individual steps
Total heat absorbed =
426 + 3174 + 3245 + 9875 + 777
= 17497 J or 17.5 kJ
benzene C6H6 vs H2O
ch1313.doc
Q. Suppose 275 g boiling hot coffee is diluted with 475 g cream at 30 oC. What is the final temperature T of the coffee.?
A. Assume coffee = cream = water (probably a very good approximation)
|
Heat lost must equal heat gained:. |
|
|
275 g x (100 - T) x 4.18 J/g oC |
= 475 g x (T - 30) x 4.18 J/g oC |
|
27500 - 275 T |
= 475 T - 14250 |
|
750 T |
=41750 |
|
T |
= 55 oC |
The answer is reasonable:
T << 100 oC and T > 30 oC
ch1313.doc
Q. Suppose 175 g of liquid water at 0oC is treated with 17.5 g superheated steam at 110 oC.
The final temperature is T?
A. Heat is conserved
|
175 g x (T - 0) x 4.18 J/g oC |
|
(warm) |
|
|
= 17.5 g x (10 oC) x 2.03 J/g oC |
(cool) |
|
|
+ 17.5 g x 2260 J/g |
(cond) |
|
|
+ 17.5 g x (100 - T) x 4.18 J/g oC |
(cool) |
|
T = 58.7 oC |
UNIT CELLS:
cubic: a crystalline form that has three equal axes at right angles to each other; isometric.
tetragonal: a crystal system characterized by three axes at right angles of which only the two lateral axes are equal
orthorhombic: crystallization characterized by three unequal axes at right angles to each other
monoclinic: crystal system characterized by three unequal axes with one oblique (not 90o) intersection
triclinic: crystal system having three unequal axes intersecting at oblique angles
rhombohedral: a parallelepiped (pairs of parallel planes) whose faces are rhombuses (equal sided parallelogram)
hexagonal: a crystal system characterized by three equal lateral axes intersecting at angles
of 60 degrees and a vertical axis of variable length at right angles
ch13defs.doc
cubic: a crystalline form that has three equal axes at right angles to each other; isometric.
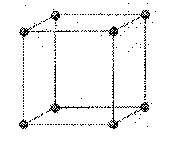
a = b = c
a
= b = g = 90o
ch13defs.doc
tetragonal: a crystal system characterized by three axes at right angles of which only the two lateral axes are equal
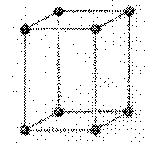
a = b ¹ c
a
= b = g = 90o
ch13defs.doc
orthorhombic: crystallization characterized by three unequal axes at right angles to each other
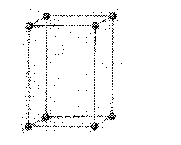
a ¹ b ¹ c
a
= b = g = 90o
ch13defs.doc
monoclinic: crystal system characterized by three unequal axes with one oblique (not 90o) intersection
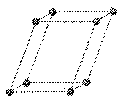
a ¹ b ¹ c
a
= g = 90o, b ¹ 90o
ch13defs.doc
triclinic: crystal system having three unequal axes intersecting at oblique angles
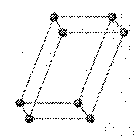
a ¹ b ¹ c
a
¹ b ¹ g ¹ 90o
ch13defs.doc
rhombohedral: a parallelepiped (pairs of parallel planes) whose faces are rhombuses (equal sided parallelogram)
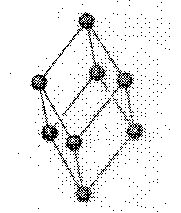
a = b = c
a
= b = g ¹ 90o
ch13defs.doc
hexagonal: a crystal system characterized by three equal lateral axes intersecting at angles
of 60 degrees and a vertical axis of variable length at right angles
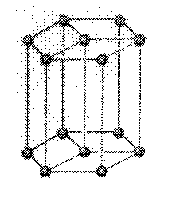
a = b ¹ c
a
= b = 90o, g = 120o
ch13face.doc
Counting atoms in cubic crystals (atoms/unit cell)
Simple Cubic
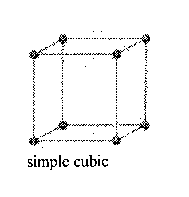
1 Atom/cell
8 corners only (corners are shared by 8 cells)
Body Centered Cubic
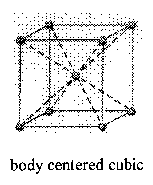
2 Atoms/cell
8 corners + 1 in the center (centers are not shared)
Face Centered Cubic
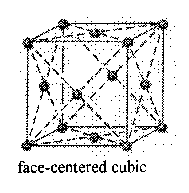
4 atoms/cell
8 corners + 6 faces (faces are shared by two cells)
ch1314.doc problem examples
Crystal density (g/cm3)
Q. Silver metal crystallizes as a solid whose lattice is face centered cubic with an edge 4.086 Å . Compute the density of silver.
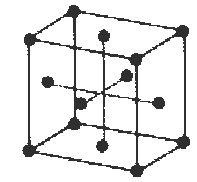
A.
mass of 1 atom of Ag
108 g x 1 mole = 1.79 x 10-22 g/atom
1 mol 6.02x1023 atoms
volume of unit cell
V = (4.086 x 10-8 cm)3
= 6.82 x 10-23 cm3
Number of Ag atoms/unit cell
8 corners = 1 atom
6 faces = 3 atoms
Total = 4 atoms/unit cell
Total mass in unit cell
4 atoms/unit cell x 1.79 x 10-22 g/atom = 7.16 x 10-22 g/unit cell
Density
D = mass/unit volume
= 7.16 x 10-22 g/unit cell = 10.5 g/cm3
6.82 x 10-23 cm3
End of notes for Chapter 13.